Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(S+O_2\underrightarrow{t^o}SO_2\)
\(1:1:1:1\)
\(0,2:0,2:0,2:0,2\left(mol\right)\)
\(n_{SO_2}=\dfrac{V}{24,79}=\dfrac{4,958}{24,79}=0,2\left(mol\right)\)
\(a,m_S=n.M=0,2.32=6,4\left(g\right)\)
\(b,V_{O_2}=n.24,79=0,2.24,79=4,958\left(l\right)\)
làm lại ko để ý có điều kiện=))))
\(n_{SO_2\left(dkc\right)}=\dfrac{V}{24,79}=\dfrac{4,958}{24,79}=0,2\left(mol\right)\)
\(PTHH:S+O_2-^{t^o}>SO_2\)
tỉ lệ 1 : 1 : 1
n(mol) 0,2<--0,2<---0,2
\(m_S=n\cdot M=0,2\cdot32=6,4\left(g\right)\\ V_{O_2\left(dkc\right)}=n\cdot24,79=0,2\cdot24,79=4,958\left(l\right)\)

a) 2Na + H2SO4 --> Na2SO4 + H2
b) \(n_{Na}=\dfrac{4,6}{23}=0,2\left(mol\right)\)
PTHH: 2Na + H2SO4 --> Na2SO4 + H2
_____0,2------>0,1-------------------->0,1
=> VH2 = 0,1.22,4 = 2,24 (l)
c) mH2SO4 = 0,1.98 = 9,8(g)

a) Mg + H2SO4 --> MgSO4 + H2
b) \(n_{Mg}=\dfrac{14,4}{24}=0,6\left(mol\right)\)
PTHH: Mg + H2SO4 --> MgSO4 + H2
0,6--->0,6------->0,6----->0,6
=> \(m_{H_2SO_4}=0,6.98=58,8\left(g\right)\)
c)
PTHH: 2H2 + O2 --to--> 2H2O
0,6-->0,3
=> VO2 = 0,3.24,79 = 7,437 (l)
=> Vkk = 7,437.5 = 37,185 (l)

Phương trình hóa học CaCO3 → CaO + CO2.
a) nCaO = 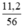 = 0,2 mol.
= 0,2 mol.
Theo PTHH thì nCaCO3 = nCaO = 0,2 (mol)
b) nCaO = 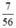 = 0,125 (mol)
= 0,125 (mol)
Theo PTHH thì nCaCO3 = nCaO = 0,125 (mol)
mCaCO3 = M.n = 100.0,125 = 12,5 (g)
c) Theo PTHH thì nCO2 = nCaCO3 = 3,5 (mol)
VCO2 = 22,4.n = 22,4.3,5 = 78,4 (lít)
d) nCO2 = 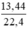 = 0,6 (mol)
= 0,6 (mol)
Theo PTHH nCaO = nCaCO3 = nCO2 = 0,6 (mol)
mCaCO3 = n.M = 0,6.100 = 60 (g)
mCaO = n.M = 0,6.56 = 33,6 (g)

PT: \(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
Ta có: \(n_{H_2}=\dfrac{3,7185}{24,79}=0,15\left(mol\right)\)
a, \(n_{Al}=\dfrac{2}{3}n_{H_2}=0,1\left(mol\right)\Rightarrow m_{Al}=0,1.27=2,7\left(g\right)\)
b, \(n_{Al_2\left(SO_4\right)_3}=\dfrac{1}{3}n_{H_2}=0,05\left(mol\right)\)
\(\Rightarrow m_{Al_2\left(SO_4\right)_3}=0,05.342=17,1\left(g\right)\)
c, \(n_{H_2SO_4}=n_{H_2}=0,15\left(mol\right)\)
\(\Rightarrow V_{H_2SO_4}=\dfrac{0,15}{1,5}=0,1\left(l\right)=100\left(ml\right)\)

PTHH: \(4P+5O_2\underrightarrow{t^o}2P_2O_5\)
a) \(n_P=\dfrac{1,24}{31}=0,04\left(mol\right)\)
Theo PTHH: \(n_{P_2O_5}=\dfrac{0,04\cdot2}{4}=0,02\left(mol\right)\)
\(\Rightarrow m_{P_2O_5}=n_{P_2O_5}\cdot M_{P_2O_5}=0,02\cdot142=2,84\left(g\right)\)
b) Theo PTHH: \(n_{O_2}=\dfrac{0,04\cdot5}{4}=0,05\left(mol\right)\)
\(\Rightarrow V_{O_2\left(dkc\right)}=n_{O_2}\cdot24,79=0,05\cdot24,79=1,2395\left(l\right)\)

\(n_{CO_2}=\dfrac{17,92}{22,4}=0,8mol\Rightarrow n_C=0,8mol\Rightarrow m_C=9,6g\)
\(n_{H_2O}=\dfrac{21,6}{18}=1,2g\Rightarrow n_H=2n_{H_2O}=2\cdot1,2=2,4mol\Rightarrow m_H=2,4g\)
\(\Rightarrow m_C+m_H=12g< m_A=18,4g\Rightarrow\)chứa O.
\(\Rightarrow m_O=18,4-12=6,4g\)
Gọi CTĐGN là \(C_xH_yO_z\)
\(x:y:z=\dfrac{m_C}{12}:\dfrac{m_H}{1}:\dfrac{m_O}{16}=\dfrac{9,6}{12}:\dfrac{2,4}{1}:\dfrac{6,4}{16}=2:6:1\)
\(\Rightarrow CTĐGN:C_2H_6O\)
a)\(C_2H_6O_2+\dfrac{5}{2}O_2\underrightarrow{t^o}2CO_2+3H_2O\)
1 0,8 1,2
\(m_{O_2}=1\cdot32=32g\)
b)Gọi CTPT là \(\left(C_2H_6O\right)_n\)
Theo bài: \(M_A=1,4375\cdot32=46\)
\(\Rightarrow46n=46\Rightarrow n=1\)
Vậy CTPT là \(C_2H_6O\)

a, \(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
b, \(n_{Al}=\dfrac{8,1}{27}=0,3\left(mol\right)\)
Theo PT: \(n_{O_2}=\dfrac{3}{4}n_{Al}=0,225\left(mol\right)\Rightarrow V_{O_2}=0,225.22,4=5,04\left(l\right)\)
c, \(2KClO_3\underrightarrow{t^o}2KCl+3O_2\)
Theo PT: \(n_{KClO_3}=\dfrac{2}{3}n_{O_2}=0,15\left(mol\right)\Rightarrow m_{KClO_3}=0,15.122,5=18,375\left(g\right)\)

Câu 1 :
$n_C = \dfrac{4,8}{12} = 0,4(mol) ; n_{O_2} = \dfrac{7,437}{24,79} = 0,3(mol)$$
$C + O_2 \xrightarrow{t^o} CO_2$
Ta thấy :
$n_C : 1 > n_{O_2} : 1$ nên C dư
$n_{C\ pư} = n_{O_2} = 0,3(mol) \Rightarrow m_{C\ dư} = (0,4 - 0,3).12 = 1,2(gam)$
$\Rightarorw V_{CO_2} = V_{O_2} = 7,437(lít)$
Câu 2 :
$n_{Mg} = \dfrac{2,4}{24} = 0,1(mol)$
$n_{Cl_2} = \dfrac{9,916}{24,79} = 0,4(mol)$
$Mg + Cl_2 \xrightarrow{t^o} MgCl_2$
Ta thấy :
$n_{Mg} : 1 < n_{Cl_2} : 1$ nên $Cl_2$ dư
$n_{Cl_2\ pư} = n_{Mg} = 0,1(mol) \Rightarrow m_{Cl_2\ dư} = (0,4 - 0,1).71 = 21,3(gam)$
$n_{MgCl_2}= n_{Mg} = 0,1(mol) \Rightarrow m_{MgCl_2} = 0,1.95 = 9,5(gam)$
`#3107.101107`
n của \(\text{CO}_2\):
\(\text{n}_{\text{CO}_2}=\dfrac{\text{V}}{24,79}=\dfrac{2,9748}{24,79}=0,12\left(\text{mol}\right)\)
PTHH: \(\text{C}+\text{O}_2\rightarrow\text{CO}_2\)
`\rightarrow`\(\text{n}_{\text{C}}=\text{n}_{\text{CO}_2}=0,12\text{ mol}\)
- Chỉ có `80%` khối lượng a tham gia vào phản ứng
m của C:
\(a=\text{n}_{\text{C}}\cdot\text{M}_{\text{C}}=\dfrac{0,12\cdot12}{80\%}=1,8\left(\text{g}\right).\)