Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a.\(n_{hh}=\dfrac{6,72}{22,4}=0,3mol\)
Gọi \(\left\{{}\begin{matrix}n_{CH_4}=x\\n_{C_2H_4}=y\end{matrix}\right.\)
\(CH_4+2O_2\rightarrow\left(t^o\right)CO_2+2H_2O\)
x x ( mol )
\(C_2H_4+3O_2\rightarrow\left(t^o\right)2CO_2+2H_2O\)
y 2y ( mol )
\(n_{CaCO_3}=\dfrac{40}{100}=0,4mol\)
\(Ca\left(OH\right)_2+CO_2\rightarrow CaCO_3+H_2O\)
0,4 0,4 ( mol )
Ta có:
\(\left\{{}\begin{matrix}x+y=0,3\\x+2y=0,4\end{matrix}\right.\) \(\Leftrightarrow\left\{{}\begin{matrix}x=0,2\\y=0,1\end{matrix}\right.\)
\(\%V_{CH_4}=\dfrac{0,2}{0,3}.100=66,67\%\)
\(\%V_{C_2H_4}=100\%-66,67\%=33,33\%\)
b.\(C_2H_4+Br_2\rightarrow C_2H_4Br_2\)
0,1 0,1 ( mol )
\(m_{Br_2}=0,1.160:10\%=160g\)

a) Khí còn lại là CH4
\(n_{CH_4} = \dfrac{3,36}{22,4} = 0,15(mol)\\ n_{C_2H_4} = \dfrac{8-0,15.16}{28} = 0,2(mol)\)
Vậy :
\(\%m_{CH_4} = \dfrac{0,15.16}{8}.100\% = 30\%\\ \%m_{C_2H_4} = 100\% - 30\% = 70\%\)
b)
\(CH_4 + 2O_2 \xrightarrow{t^o} CO_2 + 2H_2O\\ C_2H_4 + 2O_2 \xrightarrow{t^o} 2CO_2 + 2H_2O\\ CO_2 + Ca(OH)_2 \to CaCO_3 + H_2O\\ n_{CaCO_3} = n_{CO_2} = n_{CH_4} + 2n_{C_2H_4} =0,55(mol)\\ m_{CaCO_3} =0,55.100 = 55(gam) \)

a)
Khí thoát ra: CH4
\(\%V_{CH_4} = \dfrac{6,72}{16,8}.100\% = 40\%\\ \%V_{C_2H_4} = 100\% - 40\% = 60\%\)
b)
\(n_{C_2H_4} = \dfrac{16,8-6,72}{22,4} = 0,45(mol)\\ C_2H_4 + Br_2 \to C_2H_4Br_2\\ n_{Br_2} = n_{C_2H_4} = 0,45(mol)\\ \Rightarrow C_{M_{Br_2}} = \dfrac{0,45}{2} = 0,225M\\ c) n_{C_2H_4Br_2} = n_{C_2H_4} = 0,45(mol)\\ \Rightarrow n_{C_2H_4Br_2} = 0,45.188 = 84,6(gam)\)
Bài 4:
a) n(hỗn hợp khí)= 16,8/22,4=0,75(mol)
- Khí thoát ra là khí CH4.
=> nCH4=6,72/22,4=0,3(mol)
nC2H4=0,75-0,3=0,45(mol)
- Số mol tỉ lệ thuận với thể tích.
%V(CH4)=%nCH4= (0,3/0,75).100=40%
=> %V(C2H4)=100% - 40%=60%
b) PTHH: C2H4 + Br2 -> C2H4Br2
nC2H4Br2= nBr2=nC2H4=0,45(mol)
=>VddBr2= 0,45/2=0,225(l)
c) mC2H4Br2=0,45. 188= 84,6(g)

a, Ta có \(n_{CH_4}+n_{C_2H_4}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\left(1\right)\)
PT: \(CH_4+2O_2\underrightarrow{t^o}CO_2+2H_2O\)
\(C_2H_4+3O_2\underrightarrow{t^o}2CO_2+2H_2O\)
Theo PT: \(n_{O_2}=2n_{CH_4}+3n_{C_2H_4}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\left(2\right)\)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}n_{CH_4}=0,05\left(mol\right)\\n_{C_2H_4}=0,1\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}V_{CH_4}=0,05.22,4=1,12\left(l\right)\\V_{C_2H_4}=0,1.22,4=2,24\left(l\right)\end{matrix}\right.\)
b, \(m_{CH_4}=0,05.16=0,8\left(g\right)\)
c, \(C_2H_4+Br_2\rightarrow C_2H_4Br_2\)
Theo PT: \(n_{Br_2}=n_{C_2H_4}=0,1\left(mol\right)\Rightarrow V_{ddBr_2}=\dfrac{0,1}{1}=0,1\left(l\right)\)
\(m_{C_2H_4}=0,1.28=2,8\left(g\right)\)

\(n_{Br_2}=\dfrac{4}{160}=0,025\left(mol\right);n_{hh}=\dfrac{2,8}{22,4}=0,125\left(mol\right)\)
PTHH: \(C_2H_4+Br_2\rightarrow C_2H_4Br_2\)
0,025<-0,125
\(\Rightarrow\left\{{}\begin{matrix}\%V_{C_2H_4}=\dfrac{0,025}{0,125}.100\%=20\%\\\%V_{CH_4}=100\%-20\%=80\%\end{matrix}\right.\)

Ta có: \(n_{Br_2}=\dfrac{4}{160}=0,025\left(mol\right)\)
PT: \(C_2H_4+Br_2\rightarrow C_2H_4Br_2\)
Theo PT: \(n_{C_2H_4}=n_{Br_2}=0,025\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}\%V_{C_2H_4}=\dfrac{0,025.22,4}{5,6}.100\%=10\%\\\%V_{CH_4}=90\%\end{matrix}\right.\)

\(n_{Br_2}=\dfrac{8}{160}=0.05\left(mol\right)\)
\(C_2H_4+Br_2\rightarrow C_2H_4Br_2\)
\(0.05......0.05\)
\(V_{C_2H_4}=0.05\cdot22.4=1.12\left(l\right)\)
\(V_{CH_4}=20-1.12=18.88\left(l\right)\left(mol\right)\)
\(\%V_{C_2H_4}=\dfrac{1.12}{20}\cdot100\%=5.6\%\)
\(\%V_{CH_4}=100-5.6=94.4\%\)

a)
Khí còn lại là CH4
\(n_{CH_4}=\dfrac{0,448}{22,4}=0,02\left(mol\right)\)
=> \(n_{C_2H_4}=\dfrac{1,16-0,02.16}{28}=0,03\left(mol\right)\)
\(\%V_{CH_4}=\dfrac{0,02}{0,02+0,03}.100\%=40\%\)
\(\%V_{C_2H_4}=\dfrac{0,03}{0,02+0,03}.100\%=60\%\)
b)
PTHH: CH4 + 2O2 --to--> CO2 + 2H2O
0,02-------------->0,02
C2H4 + 3O2 --to--> 2CO2 + 2H2O
0,03------------->0,06
=> nCO2 = 0,02 + 0,06 = 0,08 (mol)
PTHH: Ca(OH)2 + CO2 --> CaCO3 + H2O
0,08----->0,08
=> mCaCO3 = 0,08.100 = 8 (g)

1. \(n_{Br_2}=0,4.0,5=0,2\left(mol\right)\)
PT: \(C_2H_4+Br_2\rightarrow C_2H_4Br_2\)
Theo PT: \(n_{C_2H_4}=n_{Br_2}=0,2\left(mol\right)\Rightarrow V_{C_2H_4}=0,2.22,4=4,48\left(l\right)\)
2. \(n_{C_2H_4Br_2}=\dfrac{9,4}{188}=0,05\left(mol\right)\)
PT: \(C_2H_4+Br_2\rightarrow C_2H_4Br_2\)
Theo PT: \(n_{C_2H_4}=n_{C_2H_4Br_2}=0,05\left(mol\right)\)
\(\Rightarrow\%V_{C_2H_4}=\dfrac{0,05.22,4}{1,4}.100\%=80\%\)
\(\Rightarrow\%V_{CH_4}=100-80=20\%\)
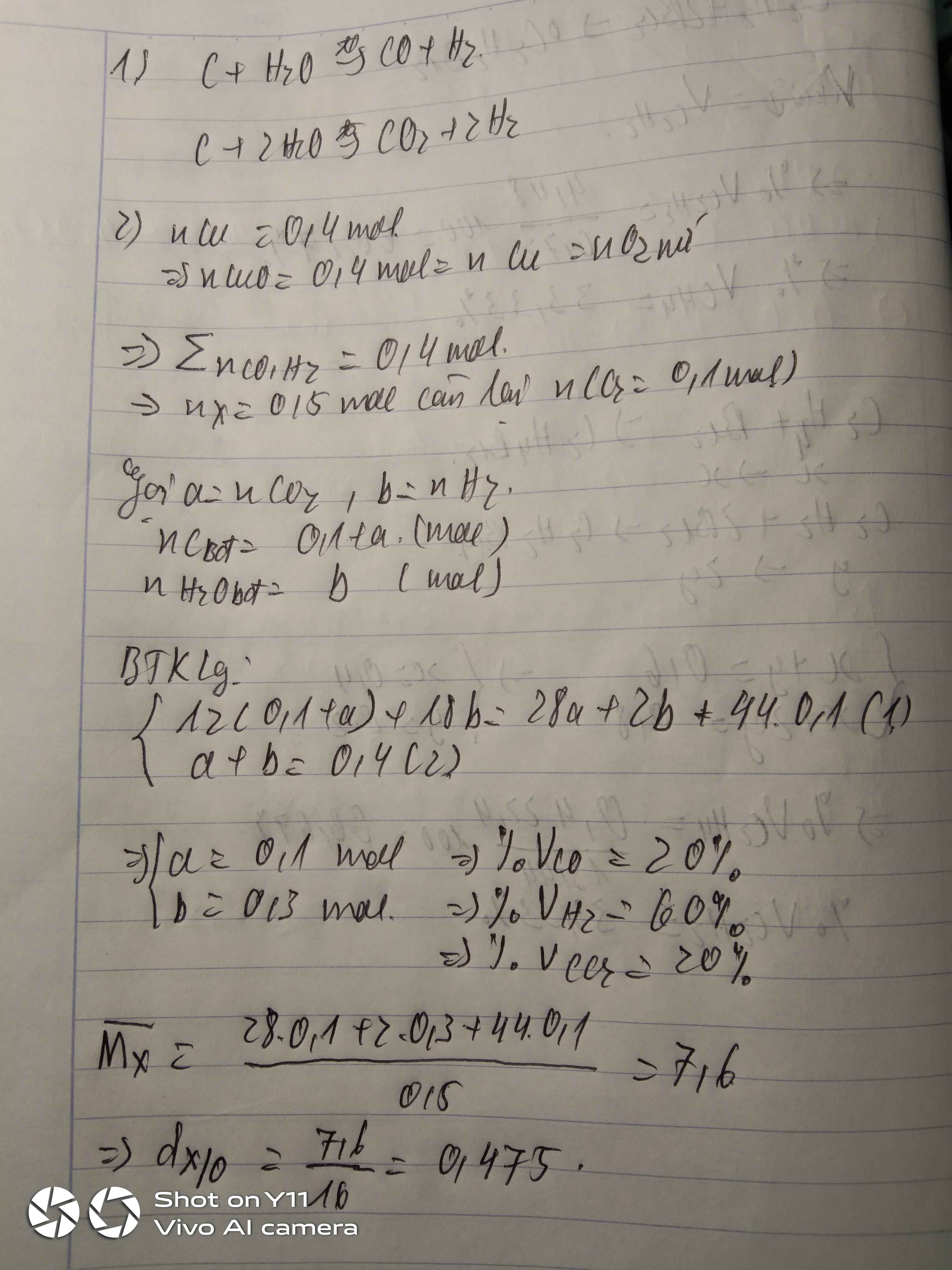
\(n_{hh}=\dfrac{7,437}{24,79}=0,3\left(mol\right)\\ C_2H_4+Br_2\rightarrow C_2H_4Br_2\\ n_{C_2H_4}=n_{C_2H_4Br_2}=\dfrac{18,8}{188}=0,1\left(mol\right)\\ \Rightarrow\%V_{\dfrac{C_2H_4}{hh}}=\dfrac{0,1}{0,3}.100\%\approx33,333\%\Rightarrow\%V_{\dfrac{CH_4}{đktc}}\approx66,667\%\)