Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(a,n_A=\dfrac{18,25}{36,5}=0,5\left(mol\right)\\ n_B=\dfrac{10,95}{36,5}=0,3\left(mol\right)\)
\(\rightarrow n_C=0,3+0,5=0,8\left(mol\right)\\ \rightarrow C_{M\left(C\right)}=\dfrac{0,8}{2}=0,4M\)
\(b,C_{M\left(A\right)}=\dfrac{0,5}{V_1}\\ C_{M\left(B\right)}=\dfrac{0,3}{V_2}\\ \rightarrow\dfrac{0,5}{V_1}:\dfrac{0,3}{V_2}=0,8\\ \rightarrow\dfrac{0,5}{V_1}=\dfrac{0,24}{V_2}=\dfrac{0,5+0,24}{V_1+V_2}=\dfrac{0,74}{2}=0,37\\ \rightarrow\left\{{}\begin{matrix}V_1=\dfrac{0,5}{0,34}=1,4\left(l\right)\\V_2=\dfrac{0,24}{0,34}=0.6\left(l\right)\end{matrix}\right.\\ \rightarrow\left\{{}\begin{matrix}C_{M\left(A\right)}=\dfrac{0,5}{1,4}=0,36M\\C_{M\left(B\right)}=\dfrac{0,5}{0,6}=0,83M\end{matrix}\right.\)

a) \(n_{HCl\left(A\right)}=\dfrac{7,3}{36,5}=0,2\left(mol\right)\)
\(n_{HCl\left(B\right)}=\dfrac{58,4}{36,5}=1,6\left(mol\right)\)
=> \(n_{HCl\left(C\right)}=0,2+1,6=1,8\left(mol\right)\)
=> \(C_{M\left(C\right)}=\dfrac{1,8}{3}=0,6M\)
b)
\(C_{M\left(A\right)}=\dfrac{0,2}{V_1}M\)
\(C_{M\left(B\right)}=\dfrac{1,6}{V_2}M\)
=> \(\dfrac{1,6}{V_2}-\dfrac{0,2}{V_1}=0,6\)
=> \(\dfrac{1,6}{3-V_1}-\dfrac{0,2}{V_1}=0,6\)
=> \(1,6.V_1-0,2\left(3-V_1\right)=0,6.V_1.\left(3-V_1\right)\)
=> \(1,6.V_1-0,6+0,2.V_1=1,8.V_1-0,6.V_1^2\)
=> \(0,6.V_1^2=0,6\)
=> V1 = 1 (l)
=> V2 = 2 (l)
\(C_{M\left(A\right)}=\dfrac{0,2}{1}=0,2M\)
\(C_{M\left(B\right)}=\dfrac{1,6}{2}=0,8M\)

a) mHCl(ddC)= 9,125+ 5,475= 14,6(g) => nHCl= 0,4(mol)
CMddHCl(ddC)= 0,4/2=0,2(M)
b) Gọi a,b lần lượt là thể thích dd HCl A và dd HCl B. (a,b>0) (lít)
nHCl(ddA)= 0,25(mol); nHCl(ddB)=0,15(mol)
Tổng thể tích ddA và dd B bằng thể tích ddC:
=>a+b=2(1)
Mặt khác: CMddA - CMddB=0,4
<=> 0,25/a - 0,15/b=0,4 (2)
Từ (1), (2) ta giải được: a=0,5 ; b=1,5
=> CMddA= 0,25/0,5=0,5(M)
CMddB=0,15/1,5=0,1(M)

a)
\(n_{Zn}=\dfrac{13}{65}=0,2\left(mol\right)\)
PTHH: Zn + 2HCl --> ZnCl2 + H2
0,2--->0,4---->0,2--->0,2
\(V_2=0,2.22,4=4,48\left(l\right)\)
\(V_1=\dfrac{0,4}{0,5}=0,8\left(l\right)\)
b)
\(C_{M\left(ZnCl_2\right)}=\dfrac{0,2}{0,8}=0,25M\)
c)
\(n_{H_2}=0,1\left(mol\right)\); \(n_{CuO}=\dfrac{32}{80}=0,4\left(mol\right)\)
PTHH: CuO + H2 --to--> Cu + H2O
Xét tỉ lệ: \(\dfrac{0,4}{1}>\dfrac{0,1}{1}\) => CuO dư, H2 hết
PTHH: CuO + H2 --to--> Cu + H2O
0,1<--0,1------>0,1
=> m = 32 - 0,1.80 + 0,1.64 = 30,4 (g)

a) \(n_{Zn}=\dfrac{13}{65}=0,2\left(mol\right)\)
PTHH: Zn + 2HCl --> ZnCl2 + H2
0,2--->0,4--->0,2--->0,2
=> V = 0,2.22,4 = 4,48 (l)
b) \(C_{M\left(dd.HCl\right)}=\dfrac{0,4}{0,5}=0,8M\)
c) \(m_{ZnCl_2}=0,2.136=27,2\left(g\right)\)

a, \(n_{Fe}=\dfrac{8,4}{56}=0,15\left(mol\right)\)
\(m_{CuCl_2}=270.10\%=27\left(g\right)\Rightarrow n_{CuCl_2}=\dfrac{27}{135}=0,2\left(mol\right)\)
Ta có: \(\dfrac{0,15}{1}< \dfrac{0,2}{1}\) ⇒ Fe hết, CuCl2 dư
PTHH: Fe + CuCl2 ---> FeCl2 + Cu
Mol: 0,15 0,15 0,15 0,15
\(a=m_{Cu}=0,15.64=9,6\left(g\right)\)
b, \(m_{dd.sau.pứ}=8,4+270-9,6=268,8\left(g\right)\)
\(m_{CuCl_2dư}=\left(0,2-0,15\right).135=6,75\left(g\right)\)
\(\left\{{}\begin{matrix}C\%_{CuCl_2dư}=\dfrac{6,75.100\%}{268,8}=2,51\%\\C\%_{FeCl_2}=\dfrac{0,15.127.100\%}{268,8}=7,09\%\end{matrix}\right.\)
c, \(V_{ddCuCl_2}=\dfrac{270}{1,35}=200\left(ml\right)=0,2\left(l\right)\)
\(\left\{{}\begin{matrix}C_{M_{CuCl_2dư}}=\dfrac{0,2-0,15}{0,2}=0,25M\\C_{M_{FeCl_2}}=\dfrac{0,15}{0,2}=0,75M\end{matrix}\right.\)

TN1:
\(C_{M\left(E\right)}=\dfrac{2x+y}{3}M\)
10ml dd E chứa \(0,01.\dfrac{2x+y}{3}\) mol H2SO4
\(n_{H_2}=\dfrac{0,05824}{22,4}=0,0026\left(mol\right)\)
PTHH: Zn + H2SO4 --> ZnSO4 + H2
=> 2x + y = 0,78 (1)
TN2:
\(C_{M\left(F\right)}=\dfrac{x+3y}{4}M\)
50ml dd F chứa \(0,05\dfrac{x+3y}{4}\) mol H2SO4
\(n_{NaOH}=\dfrac{16,8.5\%}{40}=0,021\left(mol\right)\)
PTHH: 2NaOH + H2SO4 --> Na2SO4 + 2H2O
=> x + 3y = 0,84 (2)
(1)(2) => x = 0,3; y = 0,18
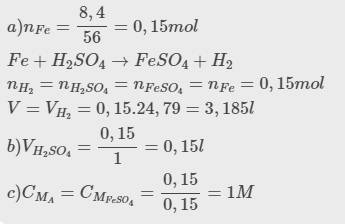

a,nA=\(\dfrac{18,25}{36,5}\)=0,5(mol)
nB=\(\dfrac{10,95}{36,5}\)=0,3(mol)
→nC=0,3+0,5=0,8(mol)
→CM(C)=\(\dfrac{0,8}{2}\)=0,4M
b,CM(A)=\(\dfrac{0,5}{V1}\)
CM(B)=\(\dfrac{0,3}{V2}\)
→\(\dfrac{0,5}{V1}\)=\(\dfrac{0,3}{V2}\)=0,8
=>V1=0,625 l
=>V2=0,375 l
=>CmV1=\(\dfrac{0,5}{0,625}\)=0,8M
=>CmV2=\(\dfrac{0,3}{0,375}\)=0,8M