
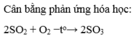
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

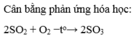

Bài 1 :
\(a) Na_2O + H_2O \to 2NaOH\\ b) N_2O_5 + H_2O \to 2HNO_3\)
Bài 2 :
\(a) Fe + 2HCl \to FeCl_2 + H_2\\ b) n_{FeCl_2} = n_{H_2} = n_{Fe} = \dfrac{11,2}{56} = 0,2(mol)\\ m_{FeCl_2} = 0,2.127 = 25,4(gam)\\ c) V_{H_2} = 0,2.22,4 = 4,48(lít)\\ n_{HCl} =2 n_{H_2} = 0,4(mol)\\ m_{HCl} = 0,4.36,5 = 14,6(gam)\)
Bài 3 :
\(n_{H_2} = \dfrac{2,24}{22,4} = 0,1(mol)\\ 2A + 2xH_2O \to 2A(OH)_x + xH_2\\ n_A = \dfrac{2}{x}n_{H_2} = \dfrac{0,2}{x}(mol)\\ \Rightarrow \dfrac{0,2}{x}.A = 4,6\\ \Rightarrow A = 23x\)
Với x = 1 thì A = 23(Natri)
Bài 4 :
Fe(H2PO4)3 : Sắt II đihidrophotphat
Zn(OH)2 : Kẽm hidroxit
H3PO3 : Axit photphoro
BaSO4 : Bari sunfat


1 a) \(2HgO\rightarrow2Hg+O_2\)
b) \(2Fe\left(OH\right)_3\rightarrow Fe_2O_3+3H_2O\)
c) \(Na_2CO_3+CaCl_2\rightarrow CaCO_3+2NaCl\)
2a) \(2Cu+O_2\rightarrow2CuO\)
b) \(N_2+3H_2\rightarrow2NH_3\)
c) \(Fe+2HCl\rightarrow FeCl_2+H_2\)
d) \(Mg\left(OH\right)_2\rightarrow MgO+H_2O\)

Bài 1:
H2 + O2 → H2O
N2O5 + H2O → HNO3
Bài 4:
Fe2(SO3)3: Sắt III sunfat
Mg(OH)2: Magie hidroxit
H3PO4: axit photphoric
Ba(HSO4)2: Bari Bisunfat
Bài 1 :
\(a.2H_2+O_2\underrightarrow{^{t^0}}2H_2O\)
\(b.N_2O_5+H_2O\rightarrow2HNO_3\)
Bài 2 :
\(n_{Al}=\dfrac{5.4}{27}=0.2\left(mol\right)\)
\(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
\(0.2............0.3...........0.1..............0.3\)
\(m_{H_2SO_4}=0.3\cdot98=29.4\left(g\right)\)
\(m_{Al_2\left(SO_4\right)_3}=0.1\cdot342=34.2\left(g\right)\)
\(m_{H_2}=0.3\cdot2=0.6\left(g\right)\)
\(V_{H_2}=0.3\cdot22.4=6.72\left(l\right)\)

\(1.4Na+O_2\rightarrow2Na_2O\)
\(2.4P+5O_2\rightarrow2P_2O_5\)
\(3.Zn+Cl_2\rightarrow ZnCl_2\)
\(4.2Al+3S\rightarrow Al_2S_3\)
\(5.2KClO_3\rightarrow2KCl+3O_2\)
\(6.2KNO_3\rightarrow2KNO_2+O_2\)
\(7.2Al\left(OH\right)_3\rightarrow Al_2O_3+3H_2O\)
\(8.3H_2+Fe_2O_3\rightarrow2Fe+3H_2O\)
\(9.3CO+Fe_2O_3\rightarrow2Fe+3CO_2\)
\(10.H_2+CuO\rightarrow Cu+H_2O\)
\(11.2Al+6HCl\rightarrow2AlCl_3+3H_2\)
\(12.Zn+2HCl\rightarrow ZnCl_2+H_2\)
\(13.2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
\(14.FeO+2HCl\rightarrow FeCl_2+H_2O\)
\(15.Na_2O+H_2O\rightarrow2NaOH\)
\(16.N_2O_5+H_2O\rightarrow2HNO_3\)
\(17.3Ca\left(OH\right)_2+2FeCl_3\rightarrow3CaCl_2+2Fe\left(OH\right)_3\)
\(18.CuCl_2+2AgNO_3\rightarrow Cu\left(NO_3\right)_2+2AgCl\)
\(19.2NaOH+FeSO_4\rightarrow Na_2SO_4+Fe\left(OH\right)_2\)
\(20.BaCl_2+H_2SO_4\rightarrow BaSO_4+2HCl\)

1) `FeO + 2HCl -> FeCl_2 + H_2O`
2) `Fe_2O_3 + 3H_2SO_4 -> Fe_2(SO_4)_3 + 3H_2O`
3) `Cu(NO_3)_2 + 2NaOH -> Cu(OH)_2 + 2NaNO_3`
4) `4P + 5O_2 -> (t^o) 2P_2O_5`
1) ���+2���−>����2+�2�FeO+2HCl−>FeCl2+H2O
2) ��2�3+3�2��4−>��2(��4)3+3�2�Fe2O3+3H2SO4−>Fe2(SO4)3+3H2O
3) ��(��3)2+2����−>��(��)2+2����3Cu(NO3)2+2NaOH−>Cu(OH)2+2NaNO3
4) 4�+5�2−>(��)2�2�54P+5O2−>(to)2P2O5