Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(a) Fe + 2HCl \to FeCl_2\\ b) n_{HCl} = \dfrac{182,5.5\%}{36,5} = 0,25(mol)\\ n_{FeCl_2} = n_{H_2} = n_{Fe} = \dfrac{1}{2}n_{HCl} = 0,125(mol)\\ \Rightarrow m_{Fe} = 0,125.56 = 7(gam) ; V = 0,125.22,4 = 2,8(lít)\\ c) m_{dd\ sau\ phản\ ứng} = m_{Fe} + m_{dd\ HCl} - m_{H_2} = 7 + 182,5 - 0,125.2 = 189,25(gam)\\ C\%_{FeCl_2} = \dfrac{0,125.127}{189,25}.100\% = 8,39\%\)

Fe+2HCl->FeCl2+H2
0,125--0,25---0,125-0,125
m HCl=9,125 g=>n HCl=\(\dfrac{9,125}{26,5}\)=0,25 mol
=>m Fe=0,125.56=7g
=>VH2=0,125.22,4=2,8l
=>C%FeCl2=\(\dfrac{0,125.127}{7+182,5-0,25}\).100=8,388%

\(n_{Zn}=\dfrac{26}{65}=0,4\left(mol\right)\\ pthh:Zn+2HCl\rightarrow ZnCl_2+H_2\uparrow\)
0,4 0,8 0,4 0,4
\(a,V_{H_2}=0,4.22,4=8,96\left(l\right)\\ b,C\%_{HCl}=\dfrac{0,8.36,5}{150}.100\%=19,5\%\\ c,m_{\text{dd}}=26+150-\left(0,4.2\right)=175,2\left(g\right)\\ C\%_{ZnCl_2}=\dfrac{0,4.136}{175,2}.100\%=31\%\)

`a)PTHH`
`Fe + 2HCl -> FeCl_2 + H_2`
`0,125` `0,25` `0,125` `0,125` `(mol)`
`n_[HCl]=[5/100 .182,5]/[36,5]=0,25(mol)`
`b)m_[Fe]=0,125.56=7(g)`
`V_[H_2]=0,125.22,4=2,8(l)`
`c)m_[HCl]=0,25.36,5=9,125(g)`
`m_[FeCl_2]=0,125.127=15,875(g)`
`d)C%_[FeCl_2]=[15,875]/[7+182,5-0,125.2] .100~~8,39%`

Mình thay trên câu a luôn nhé.
5. Số mol của Fe là :
nFe = 5,6/56 = 0,1 (mol)
a) Ta có PTHH :
Fe + 2HCl \(\rightarrow\) FeCl2 + H2\(\uparrow\)
1 mol 2 mol 1 mol 1 mol
0,1 mol 0,2 mol 0,1 mol 0,1 mol
Số mol của Fe là :
nFe = 5,6/56 = 0,1 (mol)
b) Khối lượng của FeCl2 tạo thành sau p.ứng là :
mFeCl2 = 0,1.127 = 12,7 (g)
c) Thể tích khí Hiđro (đktc) tạo thành sau p.ứng là :
VH2 = 0,1.22,4 = 2,24 (l)
4. Công thức của B là : NaxCyOz
+ \(m_{Na}=\frac{106.43,6}{100}\approx46\left(g\right)\)
\(m_C=\frac{106.11,3}{100}\approx12\left(g\right)\)
\(m_O=\frac{106.45,3}{100}\approx48\left(g\right)\)
+ \(n_{Na}=\frac{46}{23}=2\left(mol\right)\)
\(n_C=\frac{12}{12}=1\left(mol\right)\)
\(n_O=\frac{48}{16}=3\left(mol\right)\)
Suy ra trong một p.tử h/c có 2 n.tử Na, 1 n.tử C và 3 n.tử O.
\(\Rightarrow\) CTHH của hợp chất B là Na2CO3.

a, \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
b, \(n_{Zn}=\dfrac{6,5}{65}=0,1\left(mol\right)\)
\(m_{HCl}=100.14,6\%=14,6\left(g\right)\Rightarrow n_{HCl}=\dfrac{14,6}{36,5}=0,4\left(mol\right)\)
Xét tỉ lệ: \(\dfrac{0,1}{1}< \dfrac{0,4}{2}\), ta được HCl dư.
Theo PT: \(n_{H_2}=n_{Zn}=0,1\left(mol\right)\Rightarrow V_{H_2}=0,1.22,4=2,24\left(l\right)\)
b, Theo PT: \(\left\{{}\begin{matrix}n_{HCl\left(pư\right)}=2n_{Zn}=0,2\left(mol\right)\\n_{ZnCl_2}=n_{Zn}=0,1\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow n_{HCl\left(dư\right)}=0,4-0,2=0,2\left(mol\right)\)
Ta có: m dd sau pư = 6,5 + 100 - 0,1.2 = 106,3 (g)
\(\Rightarrow\left\{{}\begin{matrix}C\%_{ZnCl_2}=\dfrac{0,1.136}{106,3}.100\%\approx12,79\%\\C\%_{HCl\left(dư\right)}=\dfrac{0,2.36,5}{106,3}.100\%\approx6,87\%\end{matrix}\right.\)

nZn=0,1 mol
Zn +2HCl=> ZnCl2+ H2
0,1 mol =>0,2 mol
=>mHCl=36,5.0,2=7,3g
=>m dd HCl=7,3/14,6%=50g
mdd sau pứ=6,5+50-0,1.2=56,3g
=>C% dd ZnCl2=(0,1.136)/56,3.100%=24,16%
a.b. Zn + 2HCl ---> ZnCl2 + H2 (1)
Theo pt: 65g 73g 136g 2g
Theo đề: 6,5g 7,3g 13,6g
=> mddHCl=\(\frac{7,3.100}{14,6}=50\left(g\right)\)
c. Từ pt (1), ta có: \(C_{\%}=\frac{13,6}{50+6,5}.100\%=24,1\%\)
![]()

1)
a) Fe + 2HCl --> FeCl2 + H2
b) \(n_{Fe}=\dfrac{8,4}{56}=0,15\left(mol\right)\)
PTHH: Fe + 2HCl --> FeCl2 + H2
0,15->0,3--->0,15-->0,15
=> VH2 = 0,15.22,4 = 3,36 (l)
c) mdd sau pư = 8,4 + 250 - 0,15.2 = 258,1 (g)
=> \(C\%_{FeCl_2}=\dfrac{0,15.127}{258,1}.100\%=7,38\%\)
2)
a) Zn + 2HCl --> ZnCl2 + H2
b) \(n_{Zn}=\dfrac{13}{65}=0,2\left(mol\right)\)
PTHH: Zn + 2HCl --> ZnCl2 + H2
0,2-->0,4---->0,2--->0,2
=> VH2 = 0,2.22,4 = 4,48 (l)
mZnCl2 = 0,2.136 = 27,2 (g)
c) \(C_{M\left(dd.HCl\right)}=\dfrac{0,4}{0,2}=2M\)
d)
PTHH: A + 2HCl --> ACl2 + H2
0,2<--0,4
=> \(M_A=\dfrac{4,8}{0,2}=24\left(g/mol\right)\)
=> A là Mg(Magie)
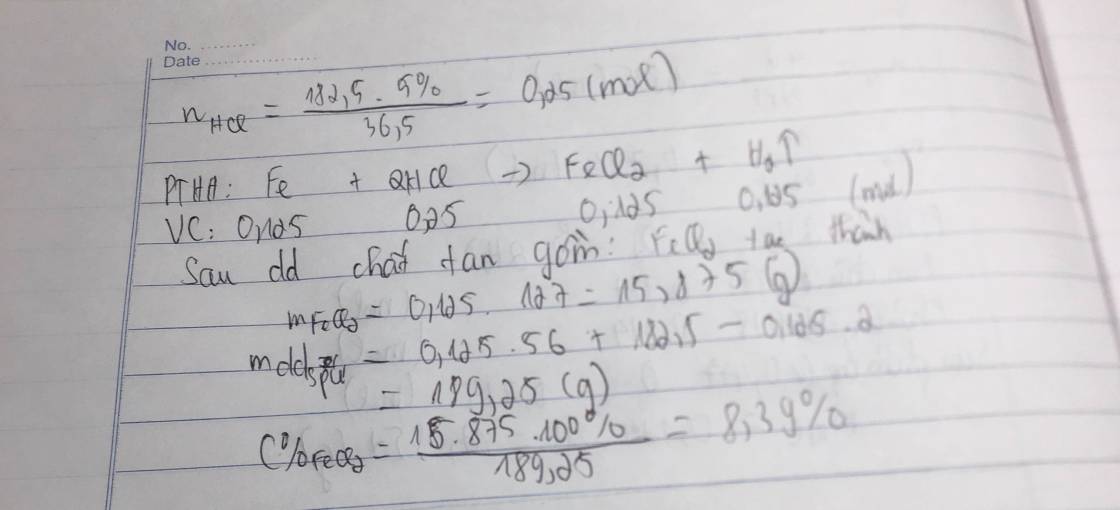
a) Fe +2HCl----->.FeCl2 +H2
Ta có
n\(_{H2}=\frac{6,72}{22,4}=0,3\left(mol\right)\)
Theo pthh
n\(_{Fe}=n_{H2}=0,3\left(mol\right)\)
m=0,3.56=16,8(g)
b) m\(_{HCl}=0,6.35,5=21,3\left(g\right)\)
m\(_{dd}=\frac{21,3.100}{7,3}=292,78\left(g\right)\)