Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{H_2}=\dfrac{2,24}{22,4}=0,1mol\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
0,1 0,2 0,1 ( mol )
\(m_{Zn}=0,1.65=6,5g\)
\(C_{MddHCl}=\dfrac{0,2}{0,4}=0,5M\)

a) nH2=V/22,4=2,24/22,4=0,1(mol)
VHCl=400ml=0,4 (lit)
PT
Zn + 2HCl -> ZnCl2 + H2
1.............2............1...........1 (mol)
0,1 < - 0,2 <- 0,1 <- 0,1 (mol)
=> a (g) = mZn=n.M=0,1.65=6,5 (g)
c) \(C_{M_{HCl}}=\dfrac{n}{V}=\dfrac{0,2}{0,4}=0,5\left(M\right)\)

a, \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
Ta có: \(n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
Theo PT: \(n_{Zn}=n_{H_2}=0,1\left(mol\right)\Rightarrow m_{Zn}=0,1.65=6,5\left(g\right)=a\)
b, \(n_{HCl}=2n_{H_2}=0,2\left(mol\right)\)
\(\Rightarrow C_{M_{HCl}}=\dfrac{0,2}{0,4}=0,5\left(M\right)\)
c, \(n_{ZnCl_2}=n_{H_2}=0,1\left(mol\right)\)
\(\Rightarrow C_{M_{ZnCl_2}}=\dfrac{0,1}{0,4}=0,25\left(M\right)\)

\(n_{Zn}=\dfrac{1,3}{65}=0,02\left(mol\right)\)
PTHH :
\(Zn+2HCl\rightarrow ZnCl_2+H_2\uparrow\)
0,02 0,04 0,02 0,02
\(V_{H_2}=0,02.22,4=0,448\left(l\right)\)
\(C_{M_{HCl}}=\dfrac{0,04}{4}=0,01M\)
b, \(CuO+H_2\underrightarrow{t^o}Cu+H_2O\)
0,02 0,02
\(m_{Cu}=0,02.64=1,28\left(g\right)\)

a, \(MgO+H_2SO_4\rightarrow MgSO_4+H_2O\)
b, Ta có: \(m_{H_2SO_4}=200.9,8\%=19,6\left(g\right)\)
\(\Rightarrow n_{H_2SO_4}=\dfrac{19,6}{98}=0,2\left(mol\right)\)
Theo PT: \(n_{MgO}=n_{MgSO_4}=n_{H_2SO_4}=0,2\left(mol\right)\)
\(\Rightarrow m_{MgO}=0,2.40=8\left(g\right)\)
c, Ta có: m dd sau pư = 8 + 200 = 208 (g)
\(\Rightarrow C\%_{MgSO_4}=\dfrac{0,2.120}{208}.100\%\approx11,54\%\)

\(n_{HCl}=0,25.2=0,5\left(mol\right)\\ a,PTHH:Zn+2HCl\rightarrow ZnCl_2+H_2\\ b,n_{Zn}=n_{H_2}=n_{ZnCl_2}=\dfrac{0,5}{2}=0,25\left(mol\right)\\ m_{Zn}=0,25.65=16,25\left(g\right)\\ c,V_{H_2\left(đktc\right)}=0,25.22,4=5,6\left(l\right)\)
Không biết đúng không nữa;-;;;
a) PTHH: Zn + 2HCl -> ZnCl2 + H2
b) HCl=250ml=0,25l
n2HCl= V/22,4= 0,5/22,4= 0,02(mol)
Zn + 2HCl -> ZnCl2 + H2
1 2 1 1
0,01 <-0,5--------------> 0,01
mZn= n.M= 0,01.65= 0,65(gam)
c) VH2=n . 22,4= 0,01 . 22,4= 0,224(l)

Fe+2HCl->FeCl2+H2
0,125--0,25---0,125-0,125
m HCl=9,125 g=>n HCl=\(\dfrac{9,125}{26,5}\)=0,25 mol
=>m Fe=0,125.56=7g
=>VH2=0,125.22,4=2,8l
=>C%FeCl2=\(\dfrac{0,125.127}{7+182,5-0,25}\).100=8,388%

\(a) Fe + 2HCl \to FeCl_2\\ b) n_{HCl} = \dfrac{182,5.5\%}{36,5} = 0,25(mol)\\ n_{FeCl_2} = n_{H_2} = n_{Fe} = \dfrac{1}{2}n_{HCl} = 0,125(mol)\\ \Rightarrow m_{Fe} = 0,125.56 = 7(gam) ; V = 0,125.22,4 = 2,8(lít)\\ c) m_{dd\ sau\ phản\ ứng} = m_{Fe} + m_{dd\ HCl} - m_{H_2} = 7 + 182,5 - 0,125.2 = 189,25(gam)\\ C\%_{FeCl_2} = \dfrac{0,125.127}{189,25}.100\% = 8,39\%\)
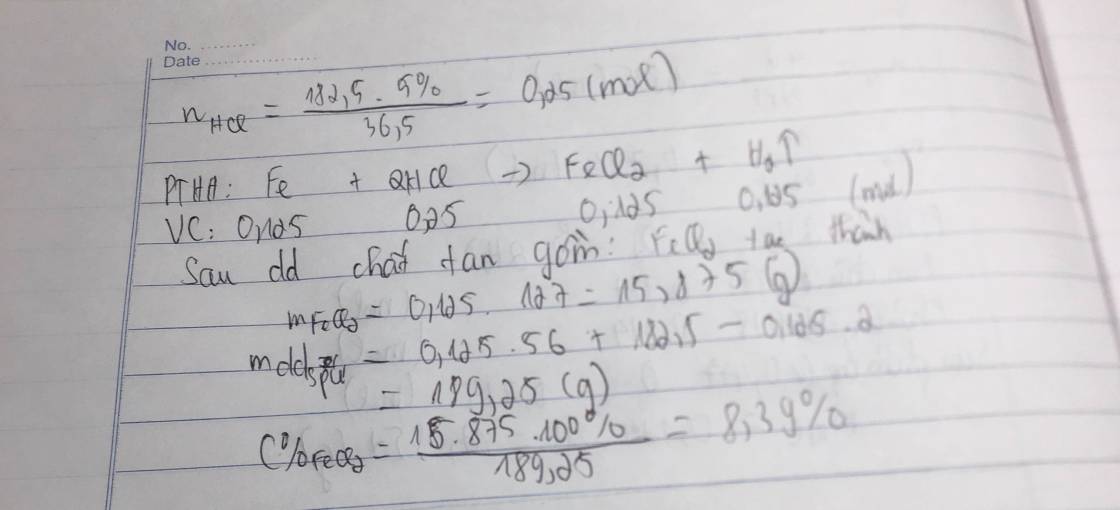
a) PTHH: Zn + 2HCl -> ZnCl2 + H2
Ta có: \(n_{H_2}=\dfrac{V_{H_2\left(đktc\right)}}{22,4}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
b) Theo PTHH và đề bài ta có:
\(n_{Zn}=n_{H_2}=0,1\left(mol\right)\)
Khối lượng Zn tham gia phản ứng:
\(a=m_{Zn}=n_{Zn}.M_{Zn}=0,1.65=6,5\left(g\right)\)
c) Ta có:
\(n_{HCl}=2.n_{H_2}=2.0,1=0,2\left(mol\right)\)
+) Ta có: \(V_{ddHCl}=400\left(ml\right)=0,4\left(l\right)\)
Nồng độ mol của ddHCl đã tham gia phản ứng:
\(C_{MddHCl}=\dfrac{n_{HCl}}{V_{ddHCl}}=\dfrac{0,2}{0,4}=0,5\left(M\right)\)