Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

1)
a) Fe + 2HCl --> FeCl2 + H2
b) \(n_{Fe}=\dfrac{8,4}{56}=0,15\left(mol\right)\)
PTHH: Fe + 2HCl --> FeCl2 + H2
0,15->0,3--->0,15-->0,15
=> VH2 = 0,15.22,4 = 3,36 (l)
c) mdd sau pư = 8,4 + 250 - 0,15.2 = 258,1 (g)
=> \(C\%_{FeCl_2}=\dfrac{0,15.127}{258,1}.100\%=7,38\%\)
2)
a) Zn + 2HCl --> ZnCl2 + H2
b) \(n_{Zn}=\dfrac{13}{65}=0,2\left(mol\right)\)
PTHH: Zn + 2HCl --> ZnCl2 + H2
0,2-->0,4---->0,2--->0,2
=> VH2 = 0,2.22,4 = 4,48 (l)
mZnCl2 = 0,2.136 = 27,2 (g)
c) \(C_{M\left(dd.HCl\right)}=\dfrac{0,4}{0,2}=2M\)
d)
PTHH: A + 2HCl --> ACl2 + H2
0,2<--0,4
=> \(M_A=\dfrac{4,8}{0,2}=24\left(g/mol\right)\)
=> A là Mg(Magie)

Fe+2HCl->FeCl2+H2
0,125--0,25---0,125-0,125
m HCl=9,125 g=>n HCl=\(\dfrac{9,125}{26,5}\)=0,25 mol
=>m Fe=0,125.56=7g
=>VH2=0,125.22,4=2,8l
=>C%FeCl2=\(\dfrac{0,125.127}{7+182,5-0,25}\).100=8,388%

\(a) Fe + 2HCl \to FeCl_2\\ b) n_{HCl} = \dfrac{182,5.5\%}{36,5} = 0,25(mol)\\ n_{FeCl_2} = n_{H_2} = n_{Fe} = \dfrac{1}{2}n_{HCl} = 0,125(mol)\\ \Rightarrow m_{Fe} = 0,125.56 = 7(gam) ; V = 0,125.22,4 = 2,8(lít)\\ c) m_{dd\ sau\ phản\ ứng} = m_{Fe} + m_{dd\ HCl} - m_{H_2} = 7 + 182,5 - 0,125.2 = 189,25(gam)\\ C\%_{FeCl_2} = \dfrac{0,125.127}{189,25}.100\% = 8,39\%\)

a) \(n_{Fe}=\dfrac{m_{Fe}}{M_{Fe}}=\dfrac{28}{56}=0,5\left(mol\right)\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
0,5-------1---------0,5------0,5
b) \(V_{H_2}=n_{H_2}.22,4=0,5.22,4=11,2\left(l\right)\)
c) \(H_2+CuO\rightarrow Cu+H_2O\)
0,5-----0,5------0,5----0,5
Khối lượng đồng tạo thành: \(m_{Cu}=n_{Cu}.64=0,5.64=32\left(g\right)\)

a) \(n_{Fe}=\dfrac{28}{56}=0,5\left(mol\right)\)
PTHH: `Fe + 2HCl -> FeCl_2 + H_2`
0,5-------------------------->0,5`
b) `V_{H_2} = 0,5.22,4 = 11,2 (l)`
c) PTHH: \(CuO+H_2\xrightarrow[]{t^o}Cu+H_2O\)
0,5---->0,5
`=> m_{Cu} = 0,5.64 = 32 (g)`
\(Fe+2HCl\underrightarrow{t^o}FeCl_2+H_2\)
\(1mol\) \(1mol\)
\(0,5mol\) \(0,5mol\)
\(n_{Fe}=\dfrac{m}{M}=\dfrac{28}{56}=0,5\left(mol\right)\)
\(V_{H_2}=n.22,4=0,5.22,4=11,2\left(l\right)\)
\(H_2+CuO\underrightarrow{t^o}Cu+H_2O\)
\(1mol\) \(1mol\)
\(0,5mol\) \(0,5mol\)
\(m_{Cu}=n.M=0,5.64=32\left(g\right)\)

a) nFe=0,1(mol); nHCl=0,4(mol)
PTHH: Fe + 2 HCl -> FeCl2 + H2
Ta có: 0,1/1 < 0,4/2
=> Fe hết, HCl dư, tish theo nFe.
b) nH2=nFeCl2=Fe=0,1(mol)
=> V(H2,đktc)=0,1.22,4=2,24(l)
c) mFeCl2=127.0,1=12,7(g)
a) nFe=0,1(mol); nHCl=0,4(mol) PTHH: Fe + 2 HCl -> FeCl2 + H2 Ta có: 0,1/1 < 0,4/2 => Fe hết, HCl dư, tish theo nFe. b) nH2=nFeCl2=Fe=0,1(mol) => V(H2,đktc)=0,1.22,4=2,24(l) c) mFeCl2=127.0,1=12,7(g)

\(a,n_{H_2}=\dfrac{1,12}{22,4}=0,05\left(mol\right)\)
PTHH: \(Fe+2HCl\rightarrow FeCl_2+H_2\uparrow\)
0,05<-0,1<------0,05<---0,05
\(b,m_{Fe}=0,05.56=2,8\left(g\right)\\ c,m_{ddHCl}=\dfrac{0,1.36,5}{14,6\%}=25\left(g\right)\\ m_{dd}=25+2,8-0,05.2=27,7\left(g\right)\\ \rightarrow C\%_{FeCl_2}=\dfrac{0,05.127}{27,7}.100\%=22,92\%\)

a. \(Fe+2HCl\rightarrow FeCl_2+H_2\)
b. \(n_{Fe}=\dfrac{m_{Fe}}{M_{Fe}}=\dfrac{28}{56}=0,5\left(mol\right)\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
0,5-------1---------0,5-----0,5
Theo PTHH: \(\Rightarrow n_{H_2}=n_{Fe}=0,5\left(mol\right)\)
\(V_{H_2}=n_{H_2}.22,4=0,5.22,4=11,2\left(l\right)\)
c. \(H_2+CuO\rightarrow Cu+H_2O\)
0,5-------0,5-----0,5----0,5
\(\Rightarrow m_{Cu}=n_{Cu}.M_{Cu}=0,5.64=32\left(g\right)\)

`a)PTHH:`
`Zn + 2HCl -> ZnCl_2 + H_2`
`0,02` `0,02` `0,02` `(mol)`
`n_[Zn]=[1,3]/65=0,02(mol)`
`b)V_[H_2]=0,02.22,4=0,448(l)`
`c)C%_[ZnCl_2]=[0,02.136]/[1,3+50-0,02.2].100~~5,31%`
\(n_{Zn}=\dfrac{1,3}{65}=0,02\left(mol\right)\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
0,02 0,04 0,02 0,02 ( mol )
\(V_{H_2}=0,02.22,4=0,448\left(l\right)\)
\(C\%_{ZnCl_2}=\dfrac{0,02.136}{1,3+50-0,02.2}.100=5,3\%\)

\(n_{Zn}=\dfrac{26}{65}=0,4\left(mol\right)\\ pthh:Zn+2HCl\rightarrow ZnCl_2+H_2\uparrow\)
0,4 0,8 0,4 0,4
\(a,V_{H_2}=0,4.22,4=8,96\left(l\right)\\ b,C\%_{HCl}=\dfrac{0,8.36,5}{150}.100\%=19,5\%\\ c,m_{\text{dd}}=26+150-\left(0,4.2\right)=175,2\left(g\right)\\ C\%_{ZnCl_2}=\dfrac{0,4.136}{175,2}.100\%=31\%\)
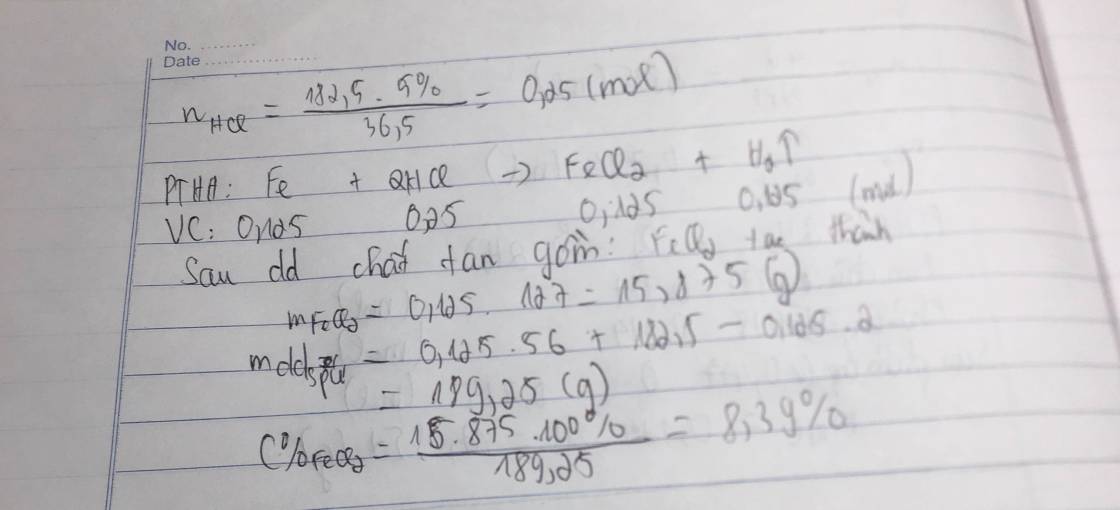
\(n_{Fe}=\dfrac{8,4}{56}=0,15\left(mol\right)\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(V_{H_2}=0,15.22,4=3,36\left(l\right)\)
\(m_{H_2}=0,15.2=0,3\left(g\right)\)
\(m_{dd}=m_{Fe}+m_{ddHCl}-m_{H_2}=8,4+250-0,3=258,1\left(g\right)\)
\(m_{FeCl_2}=0,15.127=19,05\left(g\right)\)
\(C\%_{FeCl_2}=\dfrac{19,05.100}{258,1}=7,38\%\)
`a)PTHH:`
`Fe + 2HCl -> FeCl_2 + H_2 \uparrow`
`0,15` `0,15` `0,15` `(mol)`
`n_[Fe]=[8,4]/56=0,15(mol)`
`b)V_[H_2]=0,15.22,4=3,36(l)`
`c)C%_[FeCl_2]=[0,15.127]/[8,4+250-0,15.2].100=7,38%`