Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(\left\{{}\begin{matrix}n_{Fe}=a\left(mol\right)\\n_{Cu}=b\left(mol\right)\\n_{Fe_2O_3}=c\left(mol\right)\\n_{CuO}=d\left(mol\right)\end{matrix}\right.\)⇒ 56a + 64b + 160c + 80d = 12,4(1)
BT e : \(2n_{SO_2} = 3n_{Fe} + 2n_{Cu}\)
⇒ 3a + 2b = \(2. \dfrac{2,8}{22,4} = 0,25\) ⇔ 8(3a + 2b) = 0,25.8 ⇔ 24a + 16b = 2(2)
Lấy (1) + (2),ta có :
80a + 80b + 160c + 80d = 12,4 + 2 = 14,4
Bảo toàn nguyên tố với Fe,Cu
2Fe → Fe2O3
a..............0,5a.........(mol)
Cu → CuO
b............b...............(mol)
Fe2O3 → Fe2O3
c....................c...............(mol)
CuO → CuO
d...................d................(mol)
Vậy :
\(m_Z = m_{Fe_2O_3} + m_{CuO} = 160(0,5a + c) + 80(b+d)\\ = 80a + 80b + 160c + 80d \\= 14,4(gam)\)


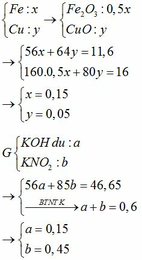
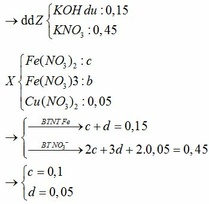

BTKL
mX + mdd HNO3 = mdd X + mH2O + m↑
=> mdd X = 11,6 + 87,5 – 30 . 0,1 – 46 . 0,15 = 89,2g
=> C%Fe(NO3)3 = 13,565%

\(n_{CuSO_4}=\dfrac{200.16}{160.100}=0,2mol\)
\(n_{NaOH}=\dfrac{200.10}{40.100}=0,5mol\)
CuSO4+2NaOH\(\rightarrow\)Cu(OH)2\(\downarrow\)+Na2SO4
-Ta có tỉ lệ: \(\dfrac{0,2}{1}< \dfrac{0,5}{2}\rightarrow\)CuSO4 hết, NaOH dư.
Cu(OH)2\(\overset{t^0}{\rightarrow}\)CuO+H2O
\(n_{CuO}=n_{Cu\left(OH\right)_2}=n_{CuSO_4}=0,2mol\)
a=\(m_{CuO}=0,2.80=16gam\)
\(m_{Cu\left(OH\right)_2}=0,2.98=19,6gam\)
\(n_{NaOH\left(pu\right)}=2n_{CuSO_4}=0,4mol\rightarrow n_{NaOH\left(dư\right)}=0,5-0,4=0,1mol\)
\(m_{NaOH\left(dư\right)}=0,1.40=4gam\)
\(n_{Na_2SO_4}=n_{CuSO_4}=0,2mol\rightarrow m_{Na_2SO_4}=0,2.136=27,2gam\)
\(m_{dd}=200+200-19,6=380,4gam\)
C%NaOH=\(\dfrac{4.100}{380,4}\approx1,05\%\)
C%Na2SO4=\(\dfrac{27,2.100}{380,4}\approx7,15\%\)

D chứa 2 oxide: \(MgO,Fe_2O_3\) (oxide 2 kim loại có tính khử cao nhất)
Vậy hỗn hợp A dư, muối đồng(II) hết.
B gồm Cu, Fe
\(Mg+CuSO_4->MgSO_4+Cu\\ Fe+CuSO_4->MgSO_4+Cu\\ MgSO_4+2NaOH->Mg\left(OH\right)_2+Na_2SO_4\\FeSO_4+2NaOH->Fe\left(OH\right)_2+Na_2SO_4 \\ Mg\left(OH\right)_2-^{^{t^{^0}}}->MgO+H_2O\\2 Fe\left(OH\right)_2+\dfrac{1}{2}O_2-^{^{ }t^{^{ }0}}->Fe_2O_3+2H_2O\\ n_{Mg}=a;n_{Fe\left(pư\right)}=b\\ \Delta m\uparrow=9,2-6,8=40a+8b=2,4\left(I\right)\\ 40a+\dfrac{160b}{2}=6\left(II\right)\\ \Rightarrow a=b=0,05mol\\ m_B=9,2=64\left(a+b\right)+56n_{Fe\left(dư\right)}\\ n_{Fe\left(dư\right)}=0,05\left(mol\right)\\ \%m_{Mg}=\dfrac{24.0,05}{6,8}.100\%=17,65\%\\ \%m_{Fe}=82,35\%\)
Bước 1: Viết các phương trình phản ứng
Phản ứng 1: Mg + CuSO4 -> MgSO4 + Cu
Phản ứng 2: Fe + CuSO4 -> FeSO4 + Cu
Phản ứng 3: Cu(OH)2 -> CuO + H2O
Bước 2: Tính toán số mol của chất rắn B
Khối lượng chất rắn B = 9,2g
Khối lượng mol CuSO4 = 63.55g/mol + 32.07g/mol + (4 * 16g/mol) = 159.62g/mol
Số mol CuSO4 = 9,2g / 159.62g/mol = 0.0577 mol
Vì phản ứng 1 và phản ứng 2 xảy ra hoàn toàn, nên số mol Mg và Fe trong hỗn hợp A cần tìm là 0.0577 mol.
Bước 3: Tính toán % số mol mỗi kim loại trong A
Khối lượng mol Mg = 24.31g/mol
Khối lượng mol Fe = 55.85g/mol
% số mol Mg trong A = (0.0577 mol * 24.31g/mol) / 6.8g * 100% = 20.34%
% số mol Fe trong A = (0.0577 mol * 55.85g/mol) / 6.8g * 100% = 47.28%
Vậy, % số mol mỗi kim loại trong hỗn hợp A là: Mg: 20.34% và Fe: 47.28%.

Bài 4 :
\(m_{ct}=\dfrac{19.120}{100}=22,8\left(g\right)\)
\(n_{MgCl2}=\dfrac{22,8}{95}=0,24\left(mol\right)\)
Pt : \(MgCl_2+Ba\left(OH\right)_2\rightarrow Mg\left(OH\right)_2+BaCl_2|\)
1 1 1 1
0,24 0,24
Pt : \(Mg\left(OH\right)_2\underrightarrow{t^o}MgO+H_2O|\)
1 1 1
0,24 0,24
\(n_{MgO}=\dfrac{0,24.1}{1}=0,24\left(mol\right)\)
⇒ \(m_{MgO}=0,24.40=9,6\left(g\right)\)
Chúc bạn học tốt

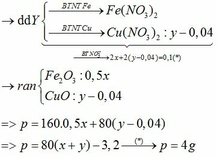
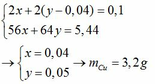
\(a)n_K=\dfrac{7,8}{39}=0,2mol\\ 2K+2H_2O\rightarrow2KOH+H_2\\ n_{H_2}=2n_K=0,4mol\\ V_{H_2\left(đktc\right)}=0,4.22,4=8,96l\\ V_{H_2\left(đkc\right)}=0,4.24,79=9,916g\\ b)n_{KOH}=n_K=0,2mol\\ 2KOH+Cu\left(NO_3\right)_2\rightarrow2KNO_3+Cu\left(OH\right)_2\\ n_{Cu\left(OH\right)_2}=\dfrac{1}{2}n_{KOH}=0,1mol\\ m_{\downarrow}=m_{Cu\left(OH\right)_2}=0,1.98=9,8g\\ c)Cu\left(OH\right)_2\xrightarrow[]{t^0}CuO+H_2O\\ n_{CuO}=n_{Cu\left(OH\right)_2}=0,1mol\\ m_{CuO}=0,1.80=8g\)