Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{Al}=\dfrac{5,4}{27}=0,2\left(mol\right)\\ n_{HCl}=\dfrac{25,55}{36,5}=0,7\left(mol\right)\\a. 2Al+6HCl\rightarrow2AlCl_3+3H_2\\ b.Vì:\dfrac{0,2}{2}< \dfrac{0,7}{6}\\ \Rightarrow HCldư\\ n_{HCl\left(dư\right)}=0,7-\dfrac{6}{2}.0,2=0,1\left(mol\right)\\ n_{AlCl_3}=n_{Al}=0,2\left(mol\right)\\ n_{H_2}=\dfrac{3}{2}.0,2=0,3\left(mol\right)\\ m_{H_2}=0,3.2=0,6\left(g\right)\\ m_{HCl\left(dư\right)}=0,1.36,5=3,65\left(g\right)\\ m_{AlCl_3}=133,5.0,2=26,7\left(g\right)\)

A) nZn=0,1(mol); nS=0,2(mol)
PTHH: Zn + S -to-> ZnS
Ta có: 0,2/1 > 0,1/1
=> Zn hết, S dư, tính theo nZnS
=> nZnS= nS(p.ứ)=nZn=0,1(mol)
=> nS(dư)=0,2-0,1=0,1(mol)
=>mS(dư)=0,1.32=3,2(g)
b) mZnS=0,1.81=8,1(g)

Câu 11:
\(n_{CaO}=\dfrac{11,2}{56}=0,2\left(mol\right);n_{H_2SO_4}=\dfrac{39,2}{98}=0,4\left(mol\right)\)
PTHH: \(CaO+H_2SO_4\rightarrow CaSO_4+H_2O\)
Ban đầu: 0,2 0,4 0,2
Sau pư: 0 0,2 0,2
`=>`\(\left\{{}\begin{matrix}m_{H_2SO_4}=0,2.98=19,6\left(g\right)\\m_{CaSO_4}=0,2.136=27,2\left(g\right)\end{matrix}\right.\)
Câu 12:
\(n_S=\dfrac{6,4}{32}=0,2\left(mol\right);n_{O_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\)
PTHH: \(S+O_2\xrightarrow[]{t^o}SO_2\)
Ban đầu: 0,2 0,5
Sau pư: 0 0,3 0,2
`=>`\(\left\{{}\begin{matrix}V_{O_2}=0,3.22,4=6,72\left(l\right)\\V_{SO_2}=0,2.22,4=4,48\left(l\right)\end{matrix}\right.\)
Câu 13:
\(n_C=\dfrac{4,8}{12}=0,4\left(mol\right);n_{O_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
PTHH: \(C+O_2\xrightarrow[]{t^o}CO_2\)
Ban đầu: 0,4 0,3
Sau pư: 0,1 0 0,3
`=>`\(\left\{{}\begin{matrix}m_{C\left(d\text{ư}\right)}=0,1.12=1,2\left(g\right)\\V_{CO_2}=0,3.22,4=6,72\left(l\right)\end{matrix}\right.\)
Câu 14:
\(n_{BaCl_2}=\dfrac{20,8}{208}=0,1\left(mol\right);n_{H_2SO_4}=\dfrac{9,8}{98}=0,1\left(mol\right)\)
PTHH: \(BaCl_2+H_2SO_4\rightarrow BaSO_4+2HCl\)
Ban đầu: 0,1 0,1
Sau pư: 0 0 0,1 0,2
`=>`\(\left\{{}\begin{matrix}m_{BaSO_4}=0,1.233=23,3\left(g\right)\\m_{HCl}=0,2.36,5=7,3\left(g\right)\end{matrix}\right.\)
Câu 15:
\(n_{CuO}=\dfrac{20}{80}=0,25\left(mol\right);n_{HCl}=\dfrac{18,25}{36,5}=0,5\left(mol\right)\)
PTHH: \(CuO+2HCl\rightarrow CuCl_2+H_2O\)
Ban đầu: 0,25 0,5
Sau pư: 0 0 0,25
`=>`\(m_{CuCl_2}=0,25.135=33,75\left(g\right)\)

a.b.\(n_S=\dfrac{m_S}{M_S}=\dfrac{6,4}{32}=0,2mol\)
\(S+O_2\rightarrow SO_2\)
0,2 0,2 0,2 ( mol )
\(V_{O_2}=n_{O_2}.22,4=0,2.22,4=4,48l\)
\(m_{SO_2}=n_{SO_2}.M_{SO_2}=0,2.64=12,8g\)
c.\(n_{H_2}=\dfrac{m_{H_2}}{M_{H_2}}=\dfrac{0,2}{2}=0,1mol\)
\(2H_2+O_2\rightarrow\left(t^o\right)2H_2O\)
0,1 < 0,2 ( mol )
0,1 0,1 ( mol )
\(m_{H_2O}=n_{H_2O}.M_{H_2O}=0,1.18=1,8g\)

\(PTHH:4Al+3O_2->2Al_2O_3\)
BĐ 0,4 0,27 (mol)
PU 0,36---->0,27---->0,18 (mol)
CL 0,04---->0------>0,18 (mol)
b)
\(n_{Al}=\dfrac{m}{M}=\dfrac{10,8}{27}=0,4\left(mol\right)\)
\(n_{O_2}=\dfrac{V}{22,4}=\dfrac{6,048}{22,4}=0,27\left(mol\right)\)
\(\dfrac{n_{Al}}{4}>\dfrac{n_{O_2}}{3}\left(\dfrac{0,4}{4}>\dfrac{0,27}{3}\right)\)
=> Al dư, O2 hết (tính theo O2)
\(m_{Al}=n\cdot M=0,04\cdot27=1,08\left(g\right)\)
c)
\(m_{Al_2O_3}=n\cdot M=0,18\cdot\left(27\cdot2+16\cdot3\right)=18,36\left(g\right)\)
a, PT: \(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
b, Ta có: \(n_{Al}=\dfrac{10,8}{27}=0,4\left(mol\right)\)
\(n_{O_2}=\dfrac{6,048}{22,4}=0,27\left(mol\right)\)
Xét tỉ lệ: \(\dfrac{0,4}{4}>\dfrac{0,27}{3}\), ta được Al dư.
Theo PT: \(n_{Al\left(pư\right)}=\dfrac{4}{3}n_{O_2}=0,36\left(mol\right)\)
\(\Rightarrow n_{Al\left(dư\right)}=0,4-0,36=0,04\left(mol\right)\)
\(\Rightarrow m_{Al\left(dư\right)}=0,04.27=1,08\left(g\right)\)
c, Theo PT: \(n_{Al_2O_3}=\dfrac{2}{3}n_{Al}=0,18\left(mol\right)\)
\(\Rightarrow m_{Al_2O_3}=0,18.102=18,36\left(g\right)\)

\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
\(n_{Al}=\dfrac{5,4}{27}=0,2\left(mol\right)\)
Theo PTHH: \(n_{AlCl_3}=n_{Al}=0,2\left(mol\right)\)
\(\Rightarrow m_{AlCl_3}=0,2.133,5=26,7\left(g\right)\)
Theo PTHH: \(n_{H_2}=\dfrac{0,2.3}{2}=0,3\left(mol\right)\)
\(\Rightarrow V_{H_2}=0,3.22,4=6,72\left(l\right)\)

\(n_{O_2}=\dfrac{3,2}{32}=0,1\left(mol\right)\)
\(n_{Al_2O_3}=\dfrac{10,2}{102}=0,1\left(mol\right)\)
PTHH :
\(4Al+3O_2\rightarrow2Al_2O_3\)
2/15 0,1 1/15
\(\dfrac{0,1}{3}< \dfrac{0,1}{2}\)
---> Tính theo O2
\(m_{Al}=\dfrac{2}{15}.27=3,6\left(g\right)\)
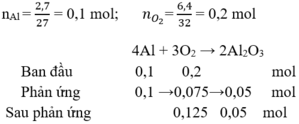
nAl = \(\dfrac{5,4}{27}=0,2\) mol
nO2 = \(\dfrac{6,4}{32}=0,2\) mol
Pt: 4Al + 3O2 --to--> 2Al2O3
0,2 mol->0,15 mol-> 0,1 mol
Xét tỉ lệ mol giữa Al và O2:
\(\dfrac{0,2}{4}< \dfrac{0,2}{3}\)
Vậy O2 dư
mAl2O3 = 0,1 . 102 = 10,2 (g)
mO2 dư = (0,2 - 0,15) . 32 = 1,6 (g)
4Al + 3O2 + 2Al2O3
0,2 0,15 0,1
nAl = m/M=5,4/27=0,2
mAl2O3= n.M=0,1×(27×2+16×3)=10,2g