Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{CuO}=\dfrac{4}{80}=0.05\left(mol\right)\)
\(n_{H_2SO_4}=0.15\cdot1=0.15\left(mol\right)\)
\(CuO+H_2SO_4\rightarrow CuSO_4+H_2O\)
\(TC:\dfrac{0.05}{1}< \dfrac{0.15}{1}\Rightarrow H_2SO_4dư\)
\(m_{CuSO_4}=0.05\cdot160=8\left(g\right)\)
\(C_{M_{CuSO_4}}=\dfrac{0.05}{0.15}=0.33\left(M\right)\)
a)
PTHH: CuO + H2SO4 -> CuSO4+ H2O
b) nCuO=0,1(mol); nH2SO4=0,15(mol)
Vì: 0,1/1 < 0,15/1
-> H2SO4 dư, CuO hết, tính theo nCuO
nCuSO4=nH2SO4(p.ứ)=nCuO=0,1(mol)
=>mCuSO4=160.0,1=16(g)
c) nH2SO4(dư)=0,05(mol)
Vddsau=VddH2SO4=0,15(l)
=>CMddH2SO4(dư)=0,05/0,15=1/3(M)
CMddCuSO4=0,1/0,15=2/3(M)

a)
$Zn + H_2SO_4 \to ZnSO_4 + H_2$
Theo PTHH :
$n_{H_2} = n_{Zn} = \dfrac{6,5}{65} = 0,1(mol)$
$V_{H_2} = 0,1.22,4 = 2,24(lít)$
b) $n_{H_2SO_4} = n_{Zn} = 0,1(mol)$
$V_{dd\ H_2SO_4} = \dfrac{0,1}{1} = 0,1(lít)$
c) $n_{ZnSO_4} = 0,1(mol) \Rightarrow m_{ZnSO_4} = 0,1.161 = 16,1(gam)$
d) $C_{M_{ZnSO_4}} = \dfrac{0,1}{0,1} = 1M$

a, \(CuO+2HCl\rightarrow CuCl_2+H_2O\)
b, \(n_{HCl}=0,2.0,5=0,1\left(mol\right)\)
Theo PT: \(n_{CuO}=n_{CuCl_2}=\dfrac{1}{2}n_{HCl}=0,05\left(mol\right)\)
\(\Rightarrow m_{CuO}=0,05.80=4\left(g\right)\)
c, \(C_{M_{CuCl_2}}=\dfrac{0,05}{0,2}=0,25\left(M\right)\)
\(n_{HCl}=0,2.0,5=0,1\left(mol\right)\)
PTHH :
\(CuO+2HCl\rightarrow CuCl_2+H_2O\)
0,05 0,1 0,05
\(b,m_{CuO}=0,05.80=4\left(g\right)\)
\(c,C_{M\left(CuCl_2\right)}=\dfrac{0,05}{0,2}=0,25\left(M\right)\)

a, \(n_{HCl}=0,15.1=0,15\left(mol_{ }\right)\)
PTHH: CuO + 2HCl → CuCl2 + H2O
Mol: 0,075 0,15 0,075
b, \(C_{M_{ddCuCl_2}}=\dfrac{0,075}{0,15}=0,5M\)

a,\(n_{CuO}=\dfrac{10}{80}=0,125\left(mol\right)\)
PTHH: CuO + 2HCl → CuCl2 + H2
Mol: 0,125 0,25 0,125
b,\(m_{CuCl_2}=0,125.135=16,875\left(g\right)\Rightarrow m_{Cu}=\dfrac{64.16,875}{135}=8\left(g\right)\)
c,\(C_{M_{ddHCl}}=\dfrac{0,25}{0,5}=0,5M\)

Bài 1:
PTHH: \(BaO+H_2SO_4\rightarrow BaSO_4+H_2O\)
Bđ____0,05___0,2
Pư____0,05___0,05_______0,05
Kt____0______0,15_______0,05
\(m_{kt}=m_{BaSO_4}=0,05.233=11,65\left(g\right)\)
\(m_{ddsaupư}=7,65+200-11,65=196\left(g\right)\)
\(C\%ddH_2SO_4=7,5\%\)
Bài 2: \(Fe_2O_3+6HCl\rightarrow2FeCl_3+3H_2O\)
bđ___0,1_______0,5
pư__1/12_______0,5_____1/6
kt ___1/60______0_______1/6
\(m_{FeCl_3}=\dfrac{1}{6}.162,5\approx27g\)
\(C_{MddFeCl_3}=\dfrac{1}{6}:0,5\approx0,3M\)

a)
$CuO + H_2SO_4 \to CuSO_4 + H_2O$
$n_{CuO} = 0,25(mol) < n_{H_2SO_4} = 0,4(mol)$ nên $H_2SO_4$ dư
$n_{CuSO_4} = n_{CuO} = 0,25(mol)$
$m_{CuSO_4} = 0,25.160 = 40(gam)$
b)
$n_{H_2SO_4\ dư} = 0,4 - 0,25 = 0,15(mol)$
$C_{M_{CuSO_4}} = \dfrac{0,25}{0,2} = 1,25M$
$C_{M_{H_2SO_4\ dư}} = \dfrac{0,15}{0,2} = 0,75M$
a) nCuO= 0,25(mol); nH2SO4= 0,4(mol)
PTHH: CuO + H2SO4 -> CuSO4 + H2O
0,25/1 < 0,4/1
=> CuO hết, H2SO4 dư, tính theo nCuO.
=> nCuSO4=nCuO=nH2SO4(p.ứ)=0,25(mol)
=> mCuSO4=0,25.160=40(g)
b) nH2SO4(dư)=0,4-0,25=0,15(mol)
Vddsau=VddH2SO4=200(ml)=0,2(l)
=>CMddCuSO4=0,25/0,2=1,25(M)
CMddH2SO4(dư)=0,15/0,2=0,75(M)
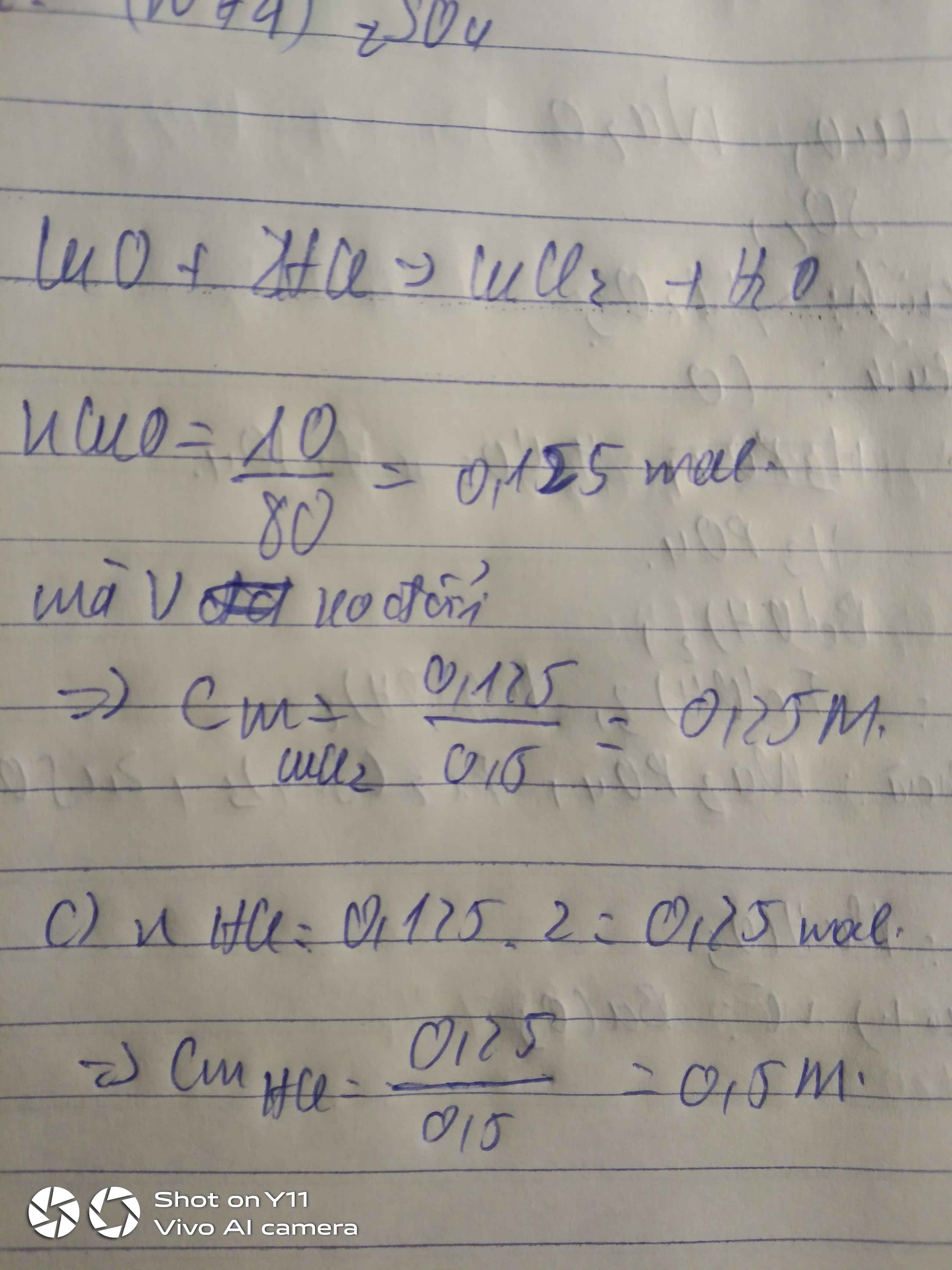
a)
$CuO + H_2SO_4 \to CuSO_4 + H_2O$
b)
$n_{CuSO_4} = n_{CuO} = \dfrac{4}{80} = 0,05(mol)$
$m_{CuSO_4} = 0,05.160 = 8(gam)$
c)
$C_{M_{CuSO_4}} = \dfrac{0,05}{0,15} = 0,33M$
cám mơn bạn