Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

PTHH: \(CuCl_2+2NaOH\rightarrow2NaCl+Cu\left(OH\right)_2\downarrow\)
\(Cu\left(OH\right)_2\xrightarrow[]{t^o}CuO+H_2O\)
Ta có: \(\left\{{}\begin{matrix}n_{CuCl_2}=0,2\cdot2=0,4\left(mol\right)\\n_{NaOH}=0,2\cdot2=0,4\left(mol\right)\end{matrix}\right.\)
Xét tỉ lệ: \(\dfrac{0,4}{1}>\dfrac{0,4}{2}\) \(\Rightarrow\) CuCl2 còn dư
\(\Rightarrow\left\{{}\begin{matrix}n_{NaCl}=0,4\left(mol\right)\\n_{Cu\left(OH\right)_2}=0,2\left(mol\right)=n_{CuO}=n_{CuCl_2\left(dư\right)}\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}m_{CuO}=0,2\cdot80=16\left(g\right)\\C_{M_{NaCl}}=\dfrac{0,4}{0,2+0,2}=1\left(M\right)\\C_{M_{CuCl_2}}=\dfrac{0,2}{0,4}=0,5\left(M\right)\\\end{matrix}\right.\)

Đáp án:
m =32,4g
mddH2SO4 = 49g
Giải thích các bước giải:
a) MgCO3 + H2SO4 → MgSO4 + H2O +CO2 ↑
MgSO4 + 2NaOH → Mg(OH)2 + Na2SO4
$Mg{(OH)_2}\buildrel {to} \over
\longrightarrow MgO + {H_2}O$
b) nCO2 = 2,24 : 22,4 = 0,1mol
nMgCO3 = nCO2 = 0,1 mol
nMgO = 12:40=0,3mol
nMgSO4 = nMgO - nMgCO3 = 0,3 - 0,1 = 0,2mol
m = mMgCO3 + mMgSO4
= 0,1 .84+0,2.120=32,4g
nH2SO4 = nCO2 = 0,1 mol
mH2SO4 = 0,1.98=9,8g
mddH2SO4 = 9,8:20.100=49g
chúc bạn học tốt

PTHH: \(CuCl_2+2NaOH\rightarrow2NaCl+Cu\left(OH\right)_2\downarrow\)
\(Cu\left(OH\right)_2\xrightarrow[]{t^o}CuO+H_2O\)
Ta có: \(n_{CuCl_2}=0,2\left(mol\right)\) \(\Rightarrow\left\{{}\begin{matrix}n_{Cu\left(OH\right)_2}=0,2\left(mol\right)=n_{CuO}\\n_{NaOH}=0,4\left(mol\right)=n_{NaCl}\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}m_{Cu\left(OH\right)_2}=0,2\cdot98=19,6\left(g\right)\\m_{CuO}=0,2\cdot80=16\left(g\right)\\m_{NaCl}=0,4\cdot58,5=23,4\left(g\right)\\C\%_{NaOH}=\dfrac{0,4\cdot40}{200}\cdot100\%=8\%\end{matrix}\right.\)

\(n_{NaOH}=\dfrac{200.10\%}{40}=0,5\left(mol\right)\)
\(PTHH:CuCl_2+2NaOH\rightarrow2NaCl+Cu\left(OH\right)_2\)
bđ: 0,3 0,5
pứ: 0,25 0,5 0,5 0,25
[ ]: 0,05 0 0,5 0,25
\(PTHH:Cu\left(OH\right)_2\underrightarrow{t^o}CuO+H_2O\)
(mol) 0,25 0,25
\(a.m_C=80.0,25=20\left(g\right)\)
\(b.m_{NaCl}=58,5.0,5=29,25\left(g\right)\\ m_{Cu\left(OH\right)_2}=0,25.98=24,5\left(g\right)\\ m_{CuCl_2\left(du\right)}=135.0,05=6,75\left(g\right)\)
\(c.m_{ddspu}=100+200-24,5=275,5\left(g\right)\\ C\%_{ddCuCl_2\left(du\right)}=\dfrac{135.0,05}{275,5}.100=2,45\left(\%\right)\\ C\%_{ddNaCl}=\dfrac{0,5.58,5}{275,25}.100=10,62\left(\%\right)\)

\(n_{KOH}=0,1.2=0,2mol\\ n_{MgSO_4}=0,1.0,8=0,08mol\\ n_{H_2SO_4}=0,1.0,4=0,04mol\)
Vì bazo và axit luôn pư trc nên H2SO4 hết MgSO4 dư.
\(2KOH+H_2SO_4\rightarrow K_2SO_4+2H_2O\)
0,08 0,04 0,04 0,08
\(2KOH+MgSO_4\rightarrow Mg\left(OH\right)_2+K_2SO_4\)
0,12 0,06 0,06 0,06
\(Mg\left(OH\right)_2\underrightarrow{t^0}MgO+H_2O\)
0,06 0,06 0,06
\(m_1=m_{Mg\left(OH\right)_2}=0,06.58=3,48g\\ m_2=m_{MgO}=0,06.40=2,4g\\ C_{M\left(K_2SO_4\right)}=\dfrac{0,04+0,06}{0,1+0,1}=0,5M\\ C_{M\left(MgSO_4\right)}=\dfrac{0,08-0,06}{0,1+0,1}=0,1M\)

a) $n_{FeCl_3} = 0,5.3 = 1,5(mol) ; n_{NaOH} = 0,3.2 = 0,6(mol)$
$FeCl_3 + 3NaOH \to Fe(OH)_3 + 3NaCl$
Ta thấy :
$n_{FeCl_3} : 1 > n_{NaOH} : 3$ nên $FeCl_3 $ dư
$n_{Fe(OH)_3} = n_{NaOH} : 3 = 0,2(mol)$
$m_{Fe(OH)_3} = 0,2.107 = 21,4(gam)$
b) $2Fe(OH)_3 \xrightarrow{t^o} Fe_2O_3 + 3H_2O$
$n_{Fe_2O_3} = \dfrac{1}{2}n_{Fe(OH)_3} = 0,1(mol)$
$a = 0,1.160 = 16(gam)$

\(a,2NaOH+MgSO_4\rightarrow Mg\left(OH\right)_2+Na_2SO_4\\ n_{NaOH}=0,5.1=0,5\left(mol\right)\\ b,n_{Mg\left(OH\right)_2}=\dfrac{0,5}{2}=0,25\left(mol\right)=n_{Na_2SO_4}\\ m_{kt}=m_{Mg\left(OH\right)_2}=58.0,25=14,5\left(g\right)\\ c,V_{ddX}=V_{ddNaOH}+V_{ddMgSO_4}=0,5+0,5=1\left(l\right)\\ C_{MddNa_2SO_4}=\dfrac{0,25}{1}=0,25\left(M\right)\)
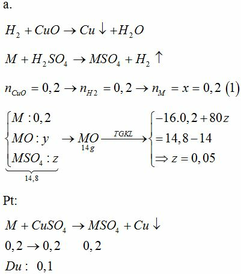
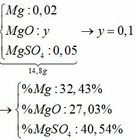

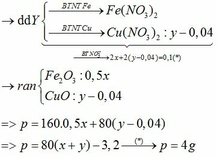
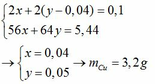
\(n_{CuSO_4}=2.0,34=0,68(mol)\\ a,CuSO_4+2NaOH\to Na_2SO_4+Cu(OH)_2\downarrow\\ Cu(OH)_2\xrightarrow{t^o}CuO+H_2O\\ \Rightarrow n_{Cu(OH)_2}=0,68(mol)\\ \Rightarrow m_{Cu(OH)_2}=0,68.98=66,64(g)\\ b,n_{CuO}=0,68(mol)\\ \Rightarrow m_{CuO}=0,68.80=54,4(g)\\ c,V_{dd_{NaOH}}=\dfrac{200}{1,25}=160(ml)\\ n_{NaOH}=\dfrac{200.32\%}{100\%.40}=1,6(mol)\)
Vì \(\dfrac{n_{CuSO_4}}{1}<\dfrac{n_{NaOH}}{2}\) nên \(NaOH\) dư
\(\Rightarrow n_{NaOH(dư)}=1,6-0,68.2=0,24(mol); n_{Na_2SO_4}=0,68(mol)\\ \Rightarrow \begin{cases} C_{M_{NaOH(dư)}}=\dfrac{0,24}{0,16}=1,5M\\ C_{M_{Na_2SO_4}}=\dfrac{0,68}{0,16}=4,25M \end{cases}\)