Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a)
\(n_{K_2O}=\dfrac{18,8}{94}=0,2\left(mol\right)\)
PTHH: K2O + H2O --> 2KOH
0,2-->0,2---->0,4
mct = 0,4.56 = 22,4 (g)
mdm = 81,2 - 0,2.18 = 77,6 (g)
mdd = 22,4 + 77,6 = 100 (g)
b)
\(C\%=\dfrac{22,4}{100}.100\%=22,4\%\)
c)
\(C\%=\dfrac{22,4}{50+100}.100\%=14,933\%\)
d)
\(m_{dd\left(sau.khi.thêm\right)}=\dfrac{22,4.100}{11,2}=200\left(g\right)\)
=> mH2O(thêm) = 200 - 100 = 100 (g)
e) Gọi khối lượng KOH thêm là x (g)
Có: \(C\%_{\left(dd.sau.khi.thêm\right)}=\dfrac{22,4+x}{100+x}.100\%=30\%\)
=> x = 10,857 (g)

a, \(n_K=\dfrac{3,9}{39}=0,1\left(mol\right)\)
PTHH: 2K + 2H2O ---> 2KOH + H2
0,1---------------->0,1----->0,05
\(m_{ct}=m_{KOH}=0,1.56=5,6\left(g\right)\\ m_{dd}=m_K+m_{H_2O}-m_{H_2}=96,2+3,9-0,05.2=100\left(g\right)\)
\(C\%_{KOH}=\dfrac{5,6}{100}.100\%=5,6\%\\ b,m_{dd}=100+50=150\left(g\right)\\ C\%_{KOH}=\dfrac{5,6}{150}.100\%=3,37\%\)
c, Gọi \(m_{H_2O}=a\left(g\right)\)
\(\Rightarrow C\%_{KOH}=\dfrac{5,6}{100+a}.100\%=2,8\%\\ \Leftrightarrow a=100\left(g\right)\)
d, Gọi \(m_{KOH}=a\left(g\right)\)
\(\Rightarrow C\%_{KOH}=\dfrac{5,6+a}{100+a}.100\%=22,4\%\\ \Leftrightarrow a=21,65\left(g\right)\)

a, Gọi \(m_{NaCl\left(thêm\right)}=a\left(g\right)\)
\(m_{NaCl\left(bđ\right)}=5\%.100=5\left(g\right)\\ \Rightarrow C\%_{NaCl}=\dfrac{5+a}{100+a}.100\%=5,5\%\\ \Leftrightarrow a=0,53\left(g\right)\)
b, \(m_{NaCl}=58,5.5,5\%=3,2175\left(g\right)\\ n_{NaCl}=\dfrac{3,2175}{58,5}=0,055\left(mol\right)\)
PTHH: NaCl + AgNO3 ---> AgCl↓ + NaNO3
0,055-->0,055------>0,055---->0,055
\(m_{AgCl}=0,055.143,5=7,8925\left(g\right)\\ m_{ddY}=58,5+200-7,8925=250,6075\left(g\right)\\ \Rightarrow C\%_{NaNO_3}=\dfrac{0,055.85}{250,6075}.100\%=1,87\%\)

\(a,C_{M\left(NaOH\right)}=\dfrac{0,3}{0,5}=0,6M\\ b,n_{NaOH}=\dfrac{24}{40}=0,6\left(mol\right)\\ C_{M\left(NaOH\right)}=\dfrac{0,6}{0,4}=1,5M\)

\(a,C\%_{KOH}=\dfrac{28}{140}.100\%=20\%\\ b,C\%_{KOH}=\dfrac{80}{80+320}.100\%=20\%\)
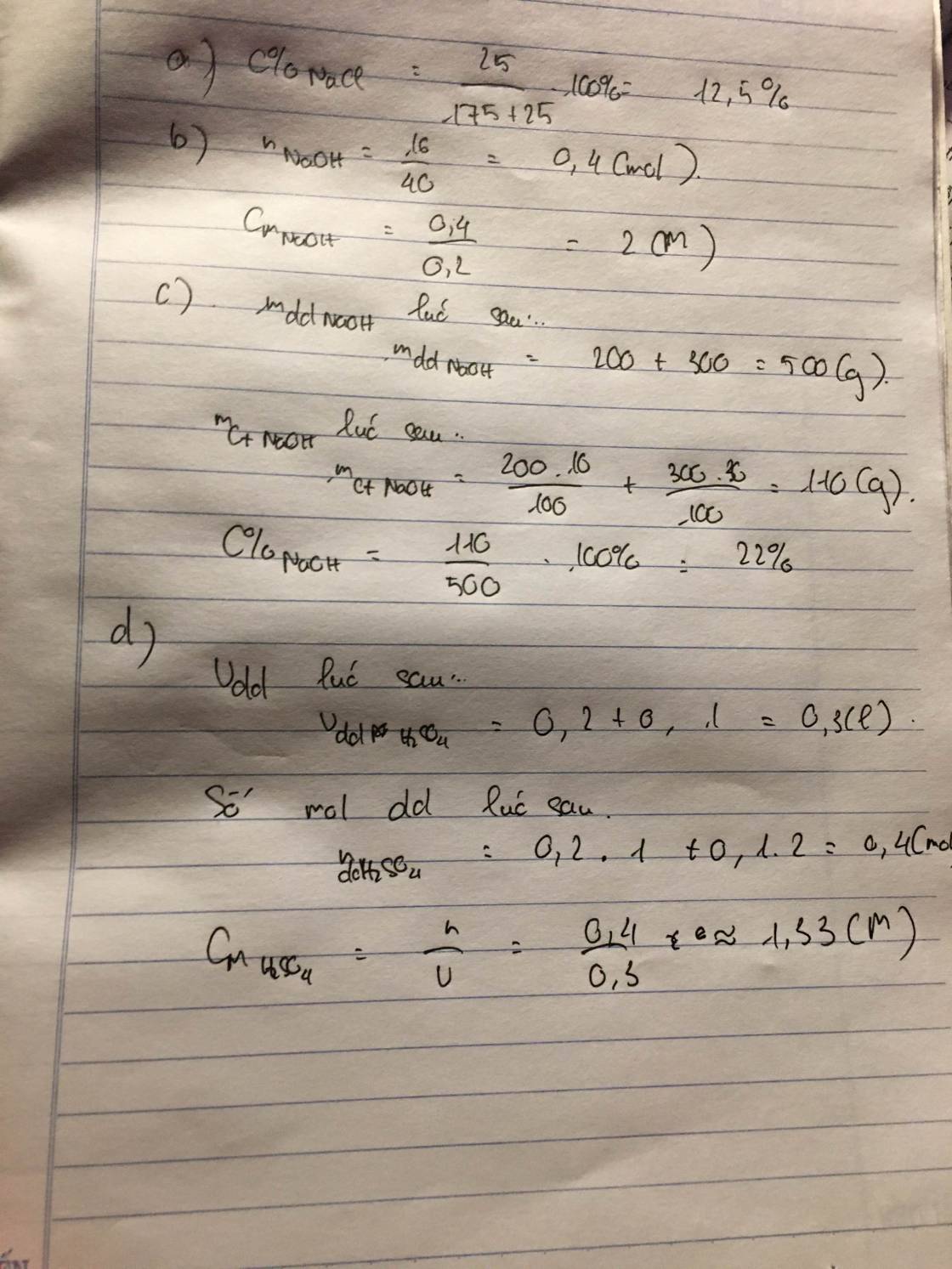
\(a,m_{ct}=30\left(g\right)\\ m_{dm}=120\left(g\right)\\ m_{dd}=120+30=150\left(g\right)\\ b,C\%_{đường}=\dfrac{30}{150}.100\%=20\%\\ c,C\%_{đường}=\dfrac{30}{150+50}.100\%=15\%\)
\(d,m_{dd}=\dfrac{30}{10\%}=300\left(g\right)\\ m_{H_2O\left(thêm\right)}=300-150=150\left(g\right)\)
e, Gọi \(m_{đường\left(thêm\right)}=a\left(g\right)\)
\(\Rightarrow C\%=\dfrac{30+a}{150+a}.100\%=30\%\\ \Leftrightarrow a=21,4285\left(g\right)\)