Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

nAL=54:27=2 mol
nAL2O3=1mol
PTHH: 4Al+3O2=>2Al2O3
2 1
2->3/2<==3/2
=> mO2=1,5.32=48g
=> V O2=1,5.22,4=33,6l

a) PTHH: \(3Fe+2O_2\underrightarrow{t^0}Fe_3O_4\left(1\right)\)
Ta có: \(n_{Fe_3O_4}=\dfrac{6,96}{232}=0,03\left(mol\right)\)
\(\Rightarrow n_{O_2}=2.0,03=0,06\left(mol\right)\)
\(\Rightarrow n_{Fe}=3.0,03=0,09\left(mol\right)\)
\(\Rightarrow m_{Fe}=0,09.56=5,04\left(mol\right)\)
b) PTHH: \(2KMnO_4\underrightarrow{t^0}K_2MnO_4+MnO_2+O_2\)
Ta có: \(n_{O_2\left(2\right)}=n_{O_2\left(1\right)}=0,06\left(mol\right)\)
\(\Rightarrow n_{KMnO_4}=2.0,06=0,12\left(mol\right)\)
\(\Rightarrow m_{KMnO_4}=0,12.158=18,96\left(g\right)\)

Bài 1:
PTHH: \(CH_4+Cl_2\underrightarrow{a/s}CH_3Cl+HCl\)
Theo PTHH: \(n_{Cl_2}=n_{CH_3Cl}=n_{CH_4}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}m_{CH_3Cl}=0,15\cdot50,5=7,575\left(g\right)\\V_{CH_4}=V_{Cl_2}=3,36\left(l\right)\end{matrix}\right.\)
Bài 2:
PTHH: \(CH_4+2O_2\underrightarrow{a/s}CO_2+2H_2O\)
Theo PTHH: \(\left\{{}\begin{matrix}n_{CO_2}=n_{CH_4}\\n_{O_2}=2n_{CH_4}\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}V_{CO_2}=V_{CH_4}=5,6\left(l\right)\\V_{CO_2}=2V_{CH_4}=11,2\left(l\right)\end{matrix}\right.\)

\(n_{Fe_3O_4}=\dfrac{23,2}{232}=0,1mol\)
\(3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\)
0,3 0,2 0,1
\(V_{O_2}=0,2\cdot22,4=4,48l\)
\(n_{Fe_3O_4}=\dfrac{23,2}{232}=0,1mol\)
\(3Fe+2O_2\rightarrow\left(t^o\right)Fe_3O_4\)
0,2 0,1 ( mol )
\(V_{O_2}=0,2.22,4=4,48l\)

\(a/n_{Fe}=\dfrac{2,52}{56}=0,045mol\\ 3Fe+2O_2\xrightarrow[]{t^0}Fe_3O_4\\ n_{O_2}=\dfrac{0,045.2}{3}=0,03mol\\ V_{O_2}=0,03.22,4=0,672l\\ b/2KClO_3\xrightarrow[]{t^0}2KCl+3O_2\\ n_{KClO_3}=\dfrac{0,03.2}{3}=0,02mol\\ m_{KClO_3}=0,02.122,5=2,45g\)

a) \(n_{Fe}=\dfrac{1,12}{56}=0,02\left(mol\right)\)
PTHH: Fe + 2HCl -->FeCl2 + H2
_____0,02->0,04--->0,02--->0,02
=> VH2 = 0,02.22,4 = 0,448(l)
b) mFeCl2 = 0,02.127 = 2,54(g)
c) \(C_{M\left(HCl\right)}=\dfrac{0,04}{0,2}=0,2M\)
Fe + 2HCl → FeCl2 + H2
1 2 1 1
0,02 0,04 0,02 0,02
nFe=\(\dfrac{1,12}{56}\)= 0,02(mol)
a). nH2=\(\dfrac{0,02.1}{1}\)= 0,02(mol)
→VH2= n . 22,4 = 0,02 . 22,4 = 0,448(l)
b). nFeCl2= \(\dfrac{0,02.1}{1}\)= 0,02(mol)
→mFeCl2= n . M = 0,02 . 127 = 2,54(g)
c). 200ml = 0,2l
nHCl= \(\dfrac{0,02.2}{1}\)=0,04(mol)
→CM= \(\dfrac{n}{V}\)= \(\dfrac{0,04}{0,2}\)= 0,2M

a) $C_2H_5OH + 3O_2 \xrightarrow{t^o} 2CO_2 + 3H_2O$
b) $n_{C_2H_5OH} = \dfrac{4,6}{46} = 0,1(mol)$
$n_{O_2} = 3n_{C_2H_5OH} = 0,3(mol)$
$V_{O_2} = 0,3.22,4 = 6,72(lít)$
c)
Theo PTHH :
$n_{CO_2} = 2n_{C_2H_5OH} = 0,2(mol) \Rightarrow V_{CO_2} = 0,2.22,4 = 4,48(lít)$
$n_{H_2O} = 3n_{C_2H_5OH} = 0,3(mol) \Rightarrow m_{H_2O} = 0,3.18 = 5,4(gam)$

\(a.Fe+2HCl\rightarrow FeCl_2+H_2\\b.n_{Fe}=\dfrac{5,6}{56}=0,1\left(mol\right)\\ n_{H_2}=n_{Fe}=0,1\left(mol\right)\\ \Rightarrow V_{H_2}=0,1.22,4=2,24\left(l\right)\\ c.n_{FeCl_2}=n_{Fe}=0,1\left(mol\right)\\ m_{FeCl_2}=0,1.127=12,7\left(g\right) \)
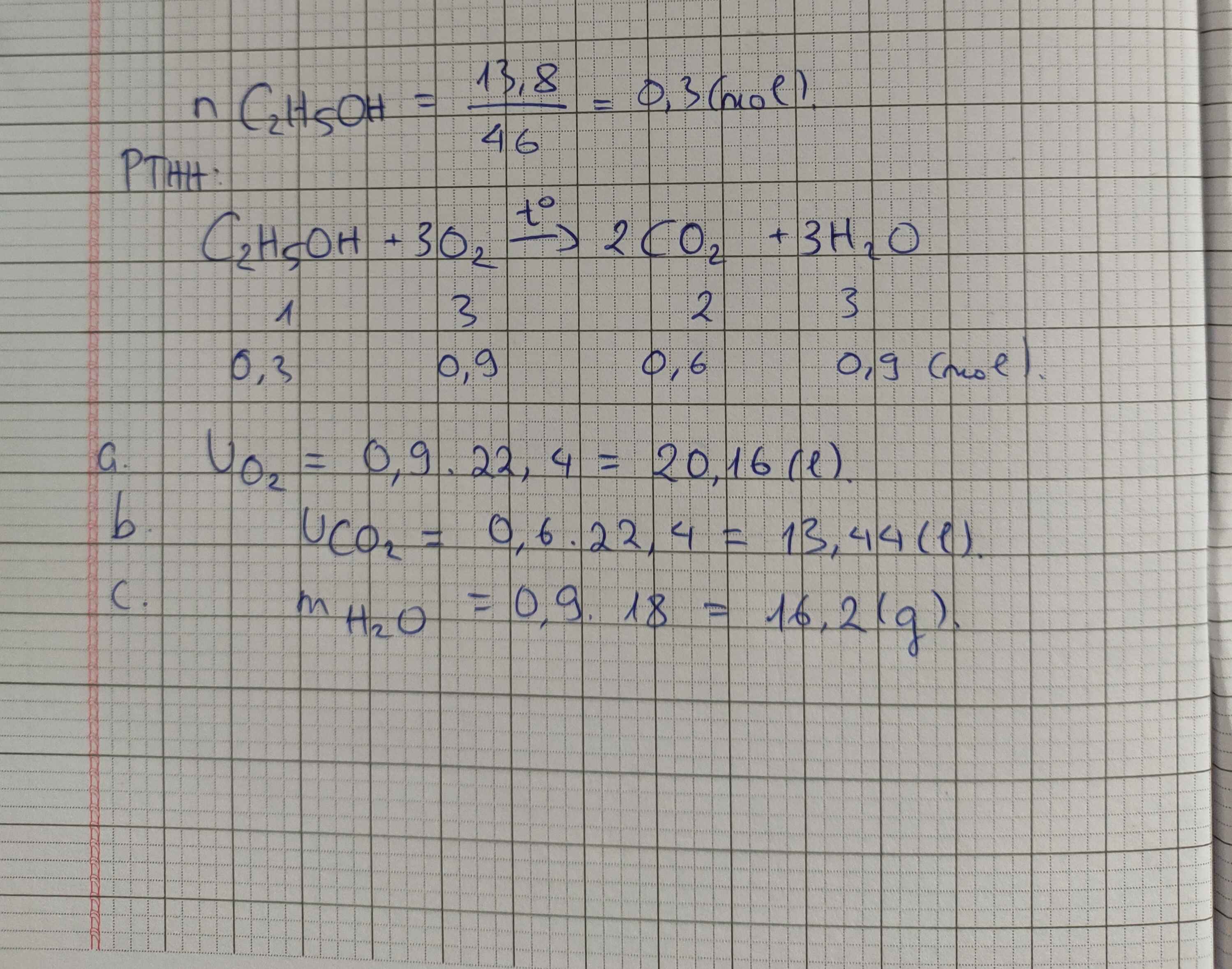
Số mol của Fe là :
\(n=\frac{m}{M}=\frac{28}{56}=0,5\) mol
PTHH : Fe + O2 -> Fe3O4
1 mol 1 mol 1mol
0,5 mol -> x = 0,5 mol -> y = 0,5 mol
a, Khối lượng oxi sắt từ thu được là :
\(m=n.M=0,5.232=116\)( g )
b, Thể tích oxi ở đktc là :
\(V=n.22,4=0,5.22,4=11,2\)( lít )