Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Trả lời:
mk chx hok wa lớp 9 nên ko giúp đc, thông cảm
HT^^
\(NaOH+HCl->NaCl+H_2O\)
a, \(m_{HCl}=\frac{C\%.m_{\text{dd}HCl}}{100\%}=\frac{7,3\%.200}{100\%}=14.6g\)
\(n_{HCl}=\frac{m_{HCl}}{M_{HCl}}=\frac{14.6}{36.5}=0.4\left(mol\right)\)
Theo PTHH ta có:\(n_{HCl}=n_{NaOH}=0.4\left(mol\right)\)
\(\Rightarrow m_{NaOH}=0,4.40=16g\)
\(\Rightarrow m_{\text{dd}NaOH}=\frac{m_{NaOH}.100\%}{C\%}=\frac{16.100\%}{10\%}=160g\)
b, Ta có \(\frac{C\%_{\text{dd}NaOH}-C\%_{\text{dd}mu\text{ối}}}{C\%_{\text{dd}mu\text{ối}}-C\%_{\text{dd}HCl}}=\frac{m_{\text{dd}HCl}}{m_{\text{dd}NaOH}}\)
\(\Leftrightarrow\frac{10\%-C\%}{C\%-7,3\%}=\frac{200}{160}=\frac{5}{4}\)\(\Rightarrow4\left(10\%-C\%\right)=5\left(C\%-7.3\%\right)\Leftrightarrow40\%-4C\%=5C\%-36.5\%\)
\(\Leftrightarrow9C\%=76.5\%\Leftrightarrow C\%=8,5\%\)

pthh: Fe+2HCl→FeCl2+H2 (1)
Theo bài ra : nH2=0,1 (mol)
theo pt(1) nFe=nH2=0,1 ⇒mFe=0,1.56=5,6 (g)
⇒ mCu=10,5-5,6=4,9 (g)
theo pt nHCl=2nH2=0,2 (Mol) ⇒mHCl=0,2.36,5=7,3(g)
C=\(\dfrac{m_{ct}}{m_{dd}}=\dfrac{7,3}{m_{dd}}\Rightarrow m_{dd}=\dfrac{7,3_{ }.100\%}{14,6\%}=50\)(g)

a) PTHH: \(Mg+2HCl\rightarrow MgCl_2+H_2\uparrow\)
b) Ta có: \(n_{Mg}=\dfrac{3,6}{24}=0,15\left(mol\right)=n_{MgCl_2}\)
\(\Rightarrow m_{MgCl_2}=0,15\cdot95=14,25\left(g\right)\)
c) Theo PTHH: \(\left\{{}\begin{matrix}n_{HCl\left(p.ứ\right)}=0,3\left(mol\right)\\n_{H_2}=0,15\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}m_{HCl\left(p.ứ\right)}=0,3\cdot36,5=10,95\left(g\right)\\m_{H_2}=0,15\cdot2=0,3\left(g\right)\end{matrix}\right.\)
Mặt khác: \(m_{dd}=m_{Mg}+m_{ddHCl}-m_{H_2}=53,3\left(g\right)\)
\(\Rightarrow m_{ddHCl}=50\left(g\right)\) \(\Rightarrow C\%_{HCl\left(p.ứ\right)}=\dfrac{10,95}{50}\cdot100\%=21,9\%\)

nZn=6,5/65=0,1mol
a, Ta có pt:Zn+2HCl--->ZnCl2+H2
b, 0,1--->0,2mol
=>thể tích HCl cần dùng là:VHCl=n/Cm=0,2/2=0,1l
Ta có pt:NaOH+HCl--->NaCl+H2
0,2<----0,2mol
mNaOH cần dùng:mNaOH=0,2.40=8g

200ml = 0,2l
\(n_{Zn}=\dfrac{19,5}{65}=0,3\left(mol\right)\)
Pt : \(Zn+2HCl\rightarrow ZnCl_2+H_2|\)
1 2 1 1
0,3 0,6 0,3 0,3
a) \(n_{ZnCl2}=\dfrac{0,3.1}{1}=0,3\left(mol\right)\)
\(C_{M_{ZnCl2}}=\dfrac{0,3}{0,2}=1,5\left(M\right)\)
b) \(n_{H2}=\dfrac{0,3.1}{1}=0,3\left(mol\right)\)
\(V_{H2\left(dktc\right)}=0,3.22,4=6,72\left(l\right)\)
c) Pt : \(NaOH+HCl\rightarrow NaCl+H_2O|\)
1 1 1 1
0,6 0,6
\(n_{NaOH}=\dfrac{0,6.1}{1}=0,6\left(mol\right)\)
\(m_{NaOH}=0,6.40=24\left(g\right)\)
\(m_{ddNaOH}=\dfrac{24.100}{20}=120\left(g\right)\)
Chúc bạn học tốt

a. PTHH:
\(BaO+2HCl--->BaCl_2+H_2O\left(1\right)\)
\(BaCO_3+2HCl--->BaCl_2+CO_2\uparrow+H_2O\left(2\right)\)
Ta có: \(n_{CO_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
Theo PT(2): \(n_{BaCO_3}=n_{CO_2}=0,2\left(mol\right)\)
\(\Rightarrow m_{BaCO_3}=0,2.197=39,4\left(g\right)\)
\(\Rightarrow\%_{m_{BaCO_3}}=\dfrac{39,4}{54,7}.100\%=72,03\%\)
\(\%_{m_{BaO}}=100\%-72,03\%=27,97\%\)
b. Ta có: \(m_{BaO}=54,7-39,4=15,3\left(g\right)\)
\(\Rightarrow n_{BaO}=\dfrac{15,3}{153}=0,1\left(mol\right)\)
\(\Rightarrow n_A=0,1+0,2=0,3\left(mol\right)\)
Theo PT(1,2): \(n_{HCl}=2.n_A=2.0,3=0,6\left(mol\right)\)
\(\Rightarrow m_{HCl}=0,6.36,5=21,9\left(g\right)\)
Ta có: \(C_{\%_{HCl}}=\dfrac{21,9}{m_{dd_{HCl}}}.100\%=20\%\)
\(\Rightarrow m_{dd_{HCl}}=109,5\left(g\right)\)
\(n_{CO2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
a) Pt : \(BaO+2HCl\rightarrow BaCl_2+H_2O|\)
1 2 1 1
0,1 0,2
\(BaCO_3+2HCl\rightarrow BaCl_2+CO_2+H_2O|\)
1 2 1 1 1
0,2 0,4 0,2
\(n_{BaCO3}=\dfrac{0,2.1}{1}=0,2\left(mol\right)\)
\(m_{BaCO3}=0,2.197=39,4\left(g\right)\)
\(m_{BaO}=54,7-39,4=15,3\left(g\right)\)
0/0BaO = \(\dfrac{15,3.100}{54,7}=27,97\)0/0
0/0BaCO3 = \(\dfrac{39,4.100}{54,7}=72,03\)0/0
b) Có : \(m_{BaO}=15,3\left(g\right)\)
\(n_{BaO}=\dfrac{15,3}{153}=0,1\left(mol\right)\)
\(n_{HCl\left(tổng\right)}=0,2+0,4=0,6\left(mol\right)\)
\(m_{HCl}=0,6.36,5=21,9\left(g\right)\)
\(m_{ddHCl}=\dfrac{21,9.100}{20}=109,5\left(g\right)\)
Chúc bạn học tốt

\(n_{H2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
a) \(Fe+2HCl\rightarrow FeCl_2+H_2|\)
1 2 1 1
0,15 0,15 0,15
b) \(n_{Fe}=\dfrac{0,15.1}{1}=0,15\left(mol\right)\)
⇒ \(m_{Fe}=01,5.56=8,4\left(g\right)\)
c) \(n_{FeCl2}=\dfrac{0,2.1}{1}=0,2\left(mol\right)\)
⇒ \(m_{FeCl2}=0,2.127=25,4\left(g\right)\)
Chúc bạn học tốt

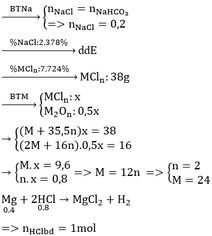
BTKL: mD + mNaHCO3 = mCO2 + mE
mD + 179,88 = 44.0,2 + 492 => mD = 320,92
BTKL: mMg + mddHCl = mH2 + mD
=> 24 . 0,4 + mddHCl = 2 . 0,4 + 320,92 => mddHCl = 312,12
=> C%HCl = 11,69%

Ừm , mình nhớ hôm qua bài này , bạn đã đăng rồi và mình cũng đã trả lời cho bạn . Bạn xem lại nhé
a) \(CaO+2HCl\rightarrow CaCl_2+H_2O\)
\(HCl+NaOH\rightarrow NaCl+H_2O\)
b) \(n_{CaO}=\dfrac{2,8}{56}=0,05\left(mol\right);n_{NaOH}=\dfrac{100.4\%}{40}=0,1\left(mol\right)\)
\(m_{muối}=m_{CaCl_2}+m_{NaCl}=0,05.111+0,1.58,5=11,4\left(g\right)\)
c) \(CM_{HCl}=\dfrac{0,05.2+0,1}{0,5}=0,4M\)