Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Lần sau bạn đăng tách từng bài ra nhé.
Câu 1:
a, \(Na_2O+H_2O\rightarrow2NaOH\)
\(n_{Na_2O}=\dfrac{15,5}{62}=0,25\left(mol\right)\)
Theo PT: \(n_{NaOH}=2n_{Na_2O}=0,5\left(mol\right)\Rightarrow C_{M_{NaOH}}=\dfrac{0,5}{0,5}=1\left(M\right)\)
b, \(2NaOH+H_2SO_4\rightarrow Na_2SO_4+2H_2O\)
Theo PT: \(n_{H_2SO_4}=\dfrac{1}{2}n_{NaOH}=0,25\left(mol\right)\)
\(\Rightarrow m_{H_2SO_4}=0,25.98=24,5\left(g\right)\)
\(\Rightarrow m_{ddH_2SO_4}=\dfrac{24,5}{20\%}=122,5\left(g\right)\)
\(\Rightarrow V_{ddH_2SO_4}=\dfrac{122,5}{1,14}\approx107,46\left(ml\right)\)
Câu 3: \(n_{CO_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\)
PT: \(CO_2+Ca\left(OH\right)_2\rightarrow CaCO_{3\downarrow}+H_2O\)
\(n_{CaCO_3}=n_{CO_2}=0,4\left(mol\right)\Rightarrow m_{CaCO_3}=0,4.100=40\left(g\right)\)
Câu 4: \(n_{CuSO_4}=\dfrac{32}{160}=0,2\left(mol\right)\)
\(n_{BaCl_2}=\dfrac{20,8}{208}=0,1\left(mol\right)\)
PT: \(CuSO_4+BaCl_2\rightarrow BaSO_{4\downarrow}+CuCl_2\)
Xét tỉ lệ: \(\dfrac{0,2}{1}>\dfrac{0,1}{1}\), ta được CuSO4 dư.
Theo PT: \(n_{BaSO_4}=n_{BaCl_2}=0,1\left(mol\right)\Rightarrow m_{BaSO_4}=0,1.233=23,3\left(g\right)\)

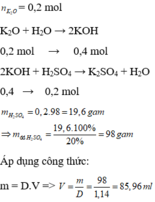
\(a,PTHH:Na_2O+H_2O\rightarrow2NaOH\\ \Rightarrow n_{NaOH}=2n_{Na_2O}=2\cdot\dfrac{37,2}{62}=0,6\cdot2=1,2\left(mol\right)\\ \Rightarrow C_{M_{NaOH}}=\dfrac{1,2}{0,5}=2,4M\\ b,PTHH:2NaOH+H_2SO_4\rightarrow Na_2SO_4+H_2O\\ \Rightarrow n_{H_2SO_4}=\dfrac{1}{2}n_{NaOH}=0,6\left(mol\right)\\ \Rightarrow m_{H_2SO_4}=0,6\cdot98=58,8\left(g\right)\\ \Rightarrow m_{dd_{H_2SO_4}}=\dfrac{58,8\cdot100\%}{20\%}=294\left(g\right)\\ \Rightarrow V_{dd}=\dfrac{294}{1,14}\approx257,9\left(ml\right)\)

a, \(Na_2O+H_2O\rightarrow2NaOH\)
Ta có: \(n_{Na_2O}=\dfrac{15,5}{62}=0,25\left(mol\right)\)
Theo PT: \(n_{NaOH}=2n_{Na_2O}=0,5\left(mol\right)\)
\(\Rightarrow CM_{NaOH}=\dfrac{0,5}{0,5}=1\left(M\right)\)
b, \(2NaOH+H_2SO_4\rightarrow Na_2SO_4+2H_2O\)
Theo PT: \(n_{H_2SO_4}=\dfrac{1}{2}n_{NaOH}=0,25\left(mol\right)\)
\(\Rightarrow m_{ddH_2SO_4}=\dfrac{0,25.98}{20\%}=122,5\left(g\right)\)
\(\Rightarrow V_{ddH_2SO_4}=\dfrac{122,5}{1,14}\approx107,46\left(ml\right)\)

\(n_{BaO}=\dfrac{30,6}{153}=0,2mol\)
\(BaO+H_2O\rightarrow Ba\left(OH\right)_2\)
0,2 0,2
Để trung hòa: \(n_{OH^-}=n_{H^+}=0,2mol\)
\(\Rightarrow m_{HCl}=0,2\cdot36,5=7,3\left(g\right)\)
\(\Rightarrow m_{ddHCl}=\dfrac{7,3}{14,6}\cdot100=50\left(g\right)\)
PTHH: BaO + H2O ---> Ba(OH)2 (1)
Ba(OH)2 + 2HCl ---> BaCl2 + 2H2O (2)
Ta có: \(n_{BaO}=\dfrac{30,6}{153}=0,2\left(mol\right)\)
Theo PT(1): \(n_{Ba\left(OH\right)_2}=n_{BaO}=0,2\left(mol\right)\)
Theo PT(2): \(n_{HCl}=2.n_{Ba\left(OH\right)_2}=2.0,2=0,4\left(mol\right)\)
=> \(m_{HCl}=0,4.36,5=14,6\left(g\right)\)
Ta có: \(C_{\%_{HCl}}=\dfrac{14,6}{m_{dd_{HCl}}}.100\%=14,6\%\)
=> \(m_{dd_{HCl}}=100\left(g\right)\)

a) PTHH: Na2O + H20 -> 2NaOH
số mol Na20 = 0,25 (mol)
=> số mol NaOH = 0,5 mol.
Nôngd độ mol NaOH = 0,5 / 0,5 = 1 M
b) PTHH: H2SO4 + 2NaOH -> Na2SO4 + 2H2O
số mol H2SO4 = 1/2 số mol NaOH = 0,25 mol
C% H2SO4 = mH2SO4 / m ddH2SO4 . 100%
=> m ddH2SO4= 122,5 g
D=m/V => V= 107,5 ml
Số mol Na2O = 15,5:62 = 0,25 mol
a) Khi cho Na2O xảy ra phản ứng, tạo thành phản ứng dung dịch có chất tan là NaOH.
Na2O + H2O → 2NaOH
Phản ứng: 0,25 → 0,05 (mol)
500 ml = = 0,5 lít; CM, NaOH =
= 1M.
b) Phương trình phản ứng trung hòa dung dịch:
2NaOH + H2SO4 → Na2SO4 + 2H2O
Phản ứng: 0, 5 → 0,25 0,25 (mol)
mH2SO4 = 0,25x98 = 24,5 g
mdd H2SO4 = = 122,5 g
mdd, ml = =
≈ 107,5 ml

nNa2O = 0,125 mol
a. Na2O + H2O --------> NaOH
0,125 mol ----------------> 0,125 mol
--> CM(NaOH) n/V = 0,125/ 0,25 = 0,5 M
b. H2SO4 + 2NaOH ------> Na2SO4 + H2O
....0,0625 <---0,125 mol
--> mH2SO4(nguyên chất) = 0,0625*98 = 6,125 g
--> mH2SO4(20%) = 6,125/20% = 30,625 g
suy ra V = m/D = 30,625 / 1,14 = 26,86 ml
nNa2O = 0,125 mol
a. Na2O + H2O --------> NaOH
0,125 mol ----------------> 0,125 mol
--> CM(NaOH) n/V = 0,125/ 0,25 = 0,5 M
b. H2SO4 + 2NaOH ------> Na2SO4 + H2O
....0,0625 <---0,125 mol
--> mH2SO4(nguyên chất) = 0,0625*98 = 6,125 g
--> mH2SO4(20%) = 6,125/20% = 30,625 g
suy ra V = m/D = 30,625 / 1,14 = 26,86 ml

a, \(Na_2O+H_2O\rightarrow2NaOH\)
\(n_{Na_2O}=\dfrac{15,5}{62}=0,25\left(mol\right)\)
Theo PT: \(n_{NaOH}=0,25.2=0,5\left(mol\right)\)
\(\Rightarrow C_{M_{NaOH}}=\dfrac{0,5}{0,5}=1\left(M\right)\)
b, \(H_2SO_4+2NaOH\rightarrow Na_2SO_4+2H_2O\)
Theo PT: \(n_{H_2SO_4}=\dfrac{1}{2}n_{NaOH}=0,25\left(mol\right)\)
\(\Rightarrow m_{H_2SO_4}=0,25.98=24,5\left(g\right)\)
\(\Rightarrow m_{ddH_2SO_4}=\dfrac{24,5}{20\%}=122,5\left(g\right)\)
\(\Rightarrow V_{ddH_2SO_4}=\dfrac{122,5}{1,14}\approx107,5\left(ml\right)\)