Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a, \(n_{Zn}=\dfrac{26}{65}=0,4\left(mol\right)\)
PT: \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
Theo PT: \(n_{H_2}=n_{Zn}=0,4\left(mol\right)\Rightarrow m_{H_2}=0,4.2=0,8\left(g\right)\)
b, \(2H_2+O_2\underrightarrow{^{t^o}}2H_2O\)
Theo PT: \(n_{O_2}=\dfrac{1}{2}n_{H_2}=0,2\left(mol\right)\Rightarrow V_{O_2}=0,2.22,4=4,48\left(l\right)\)
\(\Rightarrow V_{kk}=5V_{O_2}=22,4\left(l\right)\)

\(n_{Al}=\dfrac{2,7}{27}=0,1\left(mol\right)\\
pthh:2Al+6HCl\rightarrow2AlCl_3+3H_2\)
0,1 0,15
\(V_{H_2}=0,15.22,4=3,36l\\
pthh:2H_2O+O_2\underrightarrow{t^o}2H_2O\)
0,15 0,075
\(V_{KK}=\left(0,075.22,4\right).5=8,4l\)
\(n_{Fe_2O_3}=\dfrac{4}{160}=0,025\left(mol\right)\\
pthh:Fe_2O_3+3H_2\underrightarrow{t^o}2Fe+3H_2O\)
\(LTL:\dfrac{0,025}{1}< \dfrac{0,15}{3}\)
=> H2 dư
\(n_{Fe}=2n_{Fe_2O_3}=0,05\left(mol\right)\\
m_{Fe}=0,05.56=2,8g\)

\(n_{H_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\\ PTHH:2K+2H_2O\rightarrow2KOH+H_2\uparrow\\ Theo.pt:n_{KOH}=2n_{H_2}=2.0,15=0,3\left(mol\right)\)
\(PTHH:2KOH+H_2SO_4\rightarrow K_2SO_4+2H_2O \\ Mol:0,3\rightarrow0,15\\ V_{ddH_2SO_4}=\dfrac{0,15}{2}=0,075\left(l\right)\)

X + nH2O → X(OH)n + n/2 H2.
Ta có: n(H2) = 0,15 mol
→ n(OH-) = 2n (H2) = 0,3 mol
→ n(H+) = 0,3 mol → 2. 2V = 0,3 → V = 0,075 (lít)

C2:
PTHH: 2Al+6HCl →2AlCl3 +3H2
a)
Ta có:
\(+n_{Al}=\dfrac{8,1}{27}=0,3\left(mol\right)\)
\(+n_{HCl}=\dfrac{21,9}{36,5}=0,6\left(mol\right)\)
Biện luận:
\(\dfrac{0,3}{2}>\dfrac{0,6}{6}\)
⇒Al dư, HCl pư hết.
\(+n_{Al}\)dư =0,3-0,2=0,1(mol
\(+m_{Al}\)dư =0,1.27=2,7(gam)
b)
\(+n_{AlCl_3}=0,2\left(mol\right)\)
⇒\(m_{AlCl_3}=0,2.133,5=26,7\left(gam\right)\)
c) PTHH: H2+CuO→Cu+H2O
\(+n_{CuO}=n_{H_2}=0,3\left(mol\right)\)
\(+m_{CuO}=0,3.80=24\left(gam\right)\)
Chúc bạn học tốt.
\(1.\)
\(n_{H_2}=\dfrac{5.6}{22.4}=0.25\left(mol\right)\)
\(2N+2nHCl\rightarrow2NCl_n+nH_2\)
\(\dfrac{0.5}{n}.....0.5...............0.25\)
\(M_N=\dfrac{16.25}{\dfrac{0.5}{n}}=32.5n\left(\dfrac{g}{mol}\right)\)
\(BL:n=2\Rightarrow N=65\)
\(Nlà:Zn\)
Không tính được thể tích vì thiếu nồng độ mol nhé.
\(2.\)
\(n_{Al}=\dfrac{8.1}{27}=0.3\left(mol\right)\)
\(n_{HCl}=\dfrac{21.9}{36.5}=0.6\left(mol\right)\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
\(0.2........0.6..........0.2...........0.3\)
\(m_{Al\left(dư\right)}=\left(0.3-0.2\right)\cdot27=2.7\left(g\right)\)
\(m_{AlCl_3}=0.2\cdot133.5=26.7\left(g\right)\)
\(CuO+H_2\underrightarrow{t^0}Cu+H_2O\)
\(0.3.....0.3\)
\(m_{CuO}=0.3\cdot80=24\left(g\right)\)

a, \(n_{Zn}=\dfrac{9,75}{65}=0,15\left(mol\right)\)
PT: \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
Theo PT: \(n_{ZnCl_2}=n_{H_2}=n_{Zn}=0,15\left(mol\right)\)
\(\Rightarrow V_{H_2}=0,15.22,4=3,36\left(l\right)\)
b, \(m_{ZnCl_2}=0,15.136=20,4\left(g\right)\)

\(^nFe=\dfrac{8,4}{56}=0,15\left(mol\right)\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
mol 0,15 0,15 0,15
a) \(V_X=V_{H_2}=0,15.22,4=3,36\left(l\right)\)
b) \(^mFeCl_2=0,15.127=19,05\left(g\right)\)
Chúc bạn học tốt!!! ![]()
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
a)\(n_{Fe}=0,15mol\Rightarrow n_{M_2}=0,15mol\Rightarrow V=0,15.22,4=3,36l\)
b)\(n_{FeCl_2}=n_{Fe}=0,15mol\Rightarrow m_{muối}=0,15.127=19,05g\)

\(n_{Zn}=\dfrac{6,5}{65}=0,1\left(mol\right)\)
a. \(Zn+H_2SO_4\rightarrow ZnSO_4+H_2\)
Theo PTHH: \(n_{ZnSO_4}=n_{Zn}=0,1\left(mol\right)\)
\(\Rightarrow m_{muối}=0,1.161=16,1\left(g\right)\)
b. \(n_{H_2thu.được}=n_{Zn}=0,1\left(mol\right)\)
\(H_2+\dfrac{1}{2}O_2\underrightarrow{t^o}H_2O\)
0,1 0,05
\(V_{O_2}=0,05.22,4=1,12\left(l\right)\)
\(\Rightarrow V_{không.khí}=1,12.5=5,6\left(l\right)\)

PTHH: \(2K+2H_2O\rightarrow2KOH+H_2\)
\(n_K=\dfrac{7,8}{39}=0,2\left(mol\right)\)
Theo PTHH: \(n_{H_2}=\dfrac{1}{2}n_K=\dfrac{1}{2}.0,2=0,1\left(mol\right)\)
\(2H_2+O_2\xrightarrow[]{t^o}2H_2O\)
Theo PTHH: \(n_{O_2}=\dfrac{1}{2}n_{H_2}=\dfrac{1}{2}.0,1=0,05\left(mol\right)\)
\(\Rightarrow V_{O_2}=n_{O_2}.22.4=0,05.22,4=1,12\left(l\right)\)
Thể tích không khí đã dùng:
\(V_{kk}=\dfrac{V_{O_2}.100\%}{20\%}==5,6\left(l\right)\)
Mình cảm ơn bạn ạ bạn có thể cho mình hỏi thêm một bài nữa không ạ
Để đốt cháy 11,2g kim loại Fe trong khí Clo thủ được FeCl3
Tính thể tích khí Clo cần dùng cho phản ứng
Bạn giúp mình vs chiều nay ,1h mình thi ròi mà vẫn chưa biết làm bài này nhanh giúp mình vs ạ
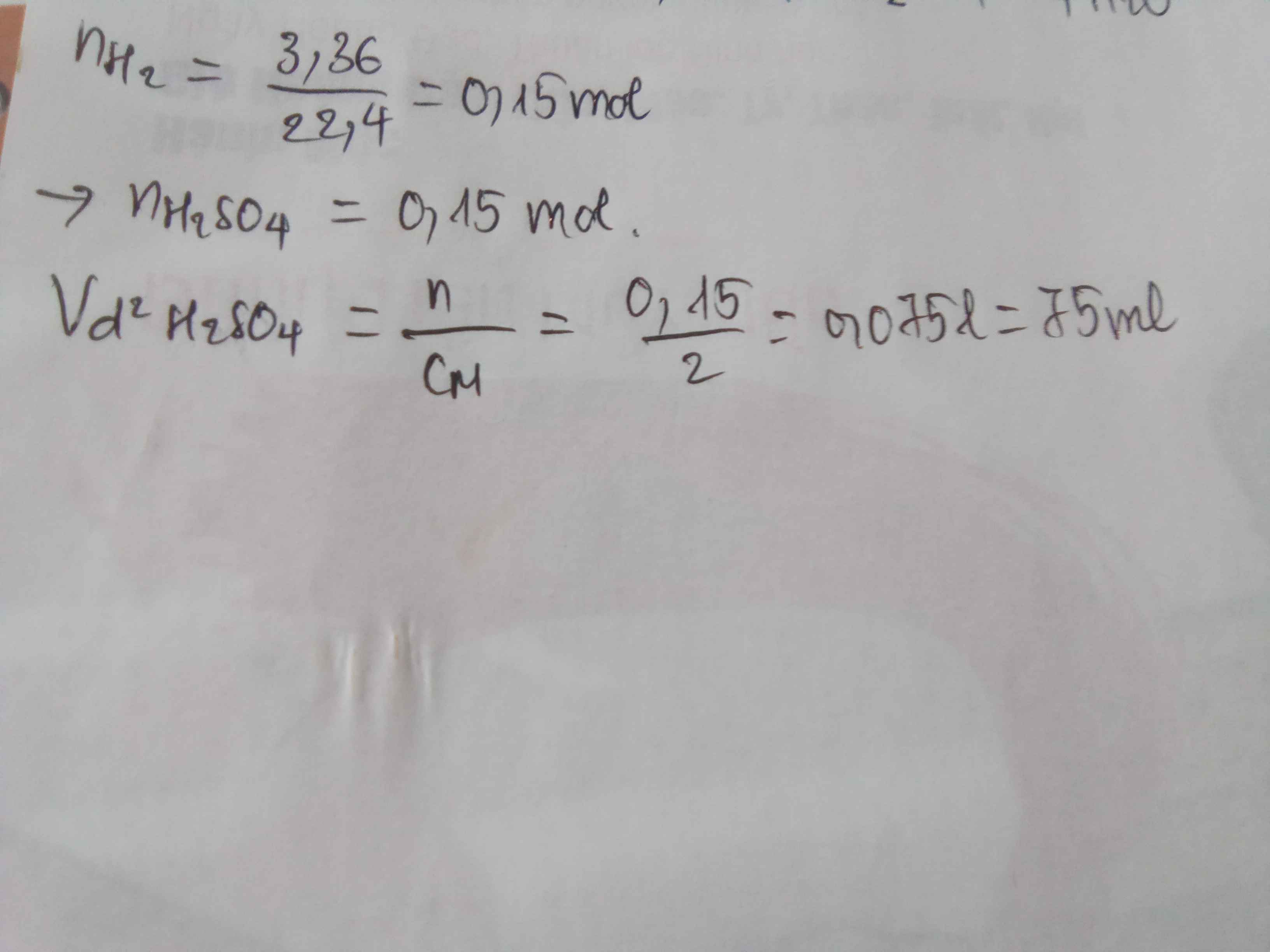
nHCl=5.10-3 mol
2Na + 2H2O --> 2NaOH + H2
x mol x mol 1/2 mol
Ba + 2H2O --> Ba(OH)2 + H2
y mol y mol y mol
NaOH + HCl --> NaCl + H2O
x mol x mol
Ba(OH)2 + 2HCl--> BaCl2 + H2O
y mol 2y mol
Ta duoc: 23x + 137y =0,297 (1)
x + 2y =5.10-3 (2)
Tu (1) va (2) ta duoc => x= 10-3
=> y= 2.10-3
a/ mNa= 10-3.23=0,023g
mBa=2.10-3.137=0,274g
b/ nH2= 10-6 mol
H2 + O2 --> H2O
10-6 mol 10-6 mol
VO2= 10-6. 22,4=2,24.10-5 lit
VKK= 2,24.10-5.100/20=1,12.10-4 lit
Phương trình chưa cân bằng kìa