Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a) S + O2 --to--> SO2
b) \(n_{SO_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\)
PTHH: S + O2 --to--> SO2
0,5<-0,5<----0,5
=> \(V_{O_2}=0,5.22,4=11,2\left(l\right)\)
c) \(m_S=0,5.32=16\left(g\right)\)

nS = 9,6/32 = 0,3 mol
S + O2 ---to----> SO2
0,3__0,3__________0,3
mSO2 = 0,3 . 64 = 19,2 (g)
VO2 = 0,3 . 22,4 = 6,72 (l)
Vkk = 6,72 . 5 = 33,6 (l)

a, 2H2 + O2 \(\underrightarrow{t^o}\) 2H2O
b, \(n_{H_2}=\dfrac{6,5}{22,4}\approx0,3\left(mol\right)\)
\(n_{O_2}=\dfrac{0,3}{2}=0,15\left(mol\right)\\ V_{O_2}=0,15.22,4=3,36\left(l\right)\)
PTHH: 2H2 + O2 -> (t°) 2H2O
Ta có: nO2 = 1/2 . nH2
=> VO2 = 1/2 . VH2 = 1/2 . 6,5 = 3,25 (l)

a, \(n_S=\dfrac{6,4}{32}=0,2\left(mol\right)\)
PTHH: S + O2 ----to----> SO2
Mol: 0,2 0,2 0,2
b, \(m_{SO_2}=0,2.64=12,8\left(g\right)\)
c, \(V_{O_2}=0,2.22,4=4,48\left(l\right)\)

Ta có: \(n_S=\dfrac{6,4}{32}=0,2\left(mol\right)\)
a. PTHH: S + O2 ---to---> SO2
Theo PT: \(n_{SO_2}=n_S=0,2\left(mol\right)\)
=> \(m_{SO_2}=0,2.64=12,8\left(g\right)\)
b. Theo PT: \(n_{O_2}=n_S=0,2\left(mol\right)\)
=> \(m_{O_2}=0,2.32=6,4\left(g\right)\)
a)S+O2-------->SO2
b)n S=6,4/32=0,2(mol)
Theo pthh
n SO2 =n S=0,2(mol)
V SO2=0,2.22,4=4,48(mol)

S+O2-to>SO2
0,2--0,2----0,2 mol
n SO2=\(\dfrac{4,48}{22,4}\)=0,2 mol
=>m S=0,2.32=6,4g
=>VO2=0,2.22,4=4,48l

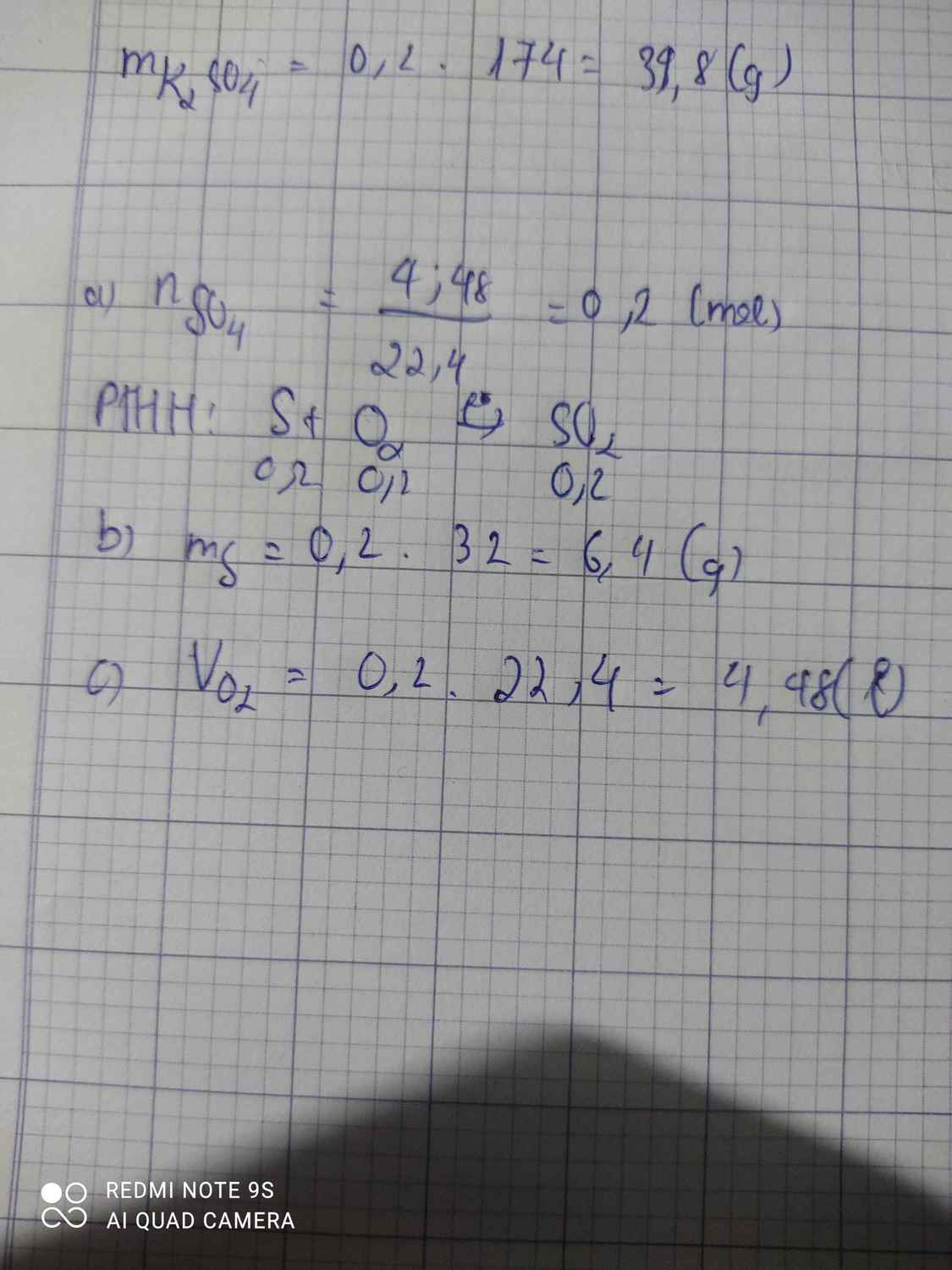
5,6 hay 6,5 :) ?
a.b.c.
\(n_{H_2}=\dfrac{V}{22,4}=\dfrac{5,6}{22,4}=0,25mol\)
\(2H_2+O_2\rightarrow\left(t^o\right)2H_2O\)
0,25 0,125 0,25 ( mol )
\(V_{O_2}=n.22,4=0,125.22,4=2,8l\)
\(m_{H_2O}=n.M=0,25.18=4,5g\)
d.
\(S+O_2\rightarrow\left(t^o\right)SO_2\)
0,125 0,125 ( mol )
\(V_{SO_2}=n.22,4=0,125.22,4=2,8l\)