Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{CH_4}=\dfrac{3,2}{16}=0,2\left(mol\right)\)
PTHH: CH4 + 2O2 --to--> CO2 + 2H2O
0,2----------------->0,2--->0,4
m1 = \(m_{CO_2}+m_{H_2O}=0,2.44+0,4.18=16\left(g\right)\)
PTHH: Ca(OH)2 + CO2 --> CaCO3 + H2O
0,2------>0,2
=> \(m_2=m_{CaCO_3}=0,2.100=20\left(g\right)\)

a, \(n_{C_2H_4}=\dfrac{1,12}{22,4}=0,05\left(mol\right)\)
PT: \(C_2H_4+3O_2\underrightarrow{t^o}2CO_2+2H_2O\)
\(CO_2+Ca\left(OH\right)_2\rightarrow CaCO_{3\downarrow}+H_2O\)
Theo PT: \(n_{CaCO_3}=n_{CO_2}=2n_{C_2H_4}=0,1\left(mol\right)\)
\(\Rightarrow m_{CaCO_3}=0,1.100=10\left(g\right)\)
b, Theo PT: \(n_{O_2}=3n_{C_2H_4}=0,15\left(mol\right)\Rightarrow V_{O_2}=0,15.22,4=3,36\left(l\right)\)
\(\Rightarrow V_{kk}=\dfrac{V_{O_2}}{20\%}=16,8\left(l\right)\)

\(n_{CH_4}=\dfrac{28}{22.4}=1.25\left(mol\right)\)
\(CH_4+2O_2\rightarrow CO_2+2H_2O\)
\(1.25.................1.25.......2.5\)
\(m_{\text{bình tăng}}=m_{CO_2}+m_{H_2O}=1.25\cdot44+2.5\cdot18=100\left(g\right)\)
\(CO_2+Ca\left(OH\right)_2\rightarrow CaCO_3+H_2O\)
\(1.25...............................1.25\)
\(m_{CaCO_3}=1.25\cdot100=125\left(\right)\)

\(n_{C_2H_4}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\\ C_2H_4+3O_2\rightarrow\left(t^o\right)2CO_2+2H_2O\\ n_{CO_2}=2.n_{C_2H_4}=2.0,5=1\left(mol\right)\\ CO_2+Ca\left(OH\right)_2\rightarrow CaCO_3+H_2O\\ n_{CaCO_3}=n_{CO_2}=1\left(mol\right)\\ m_{kết.tủa}=m_{CaCO_3}=100.1=100\left(g\right)\\ n_{O_2}=3.n_{C_2H_4}=3.0,5=1,5\left(mol\right)\\ V_{kk}=\dfrac{100}{20}.V_{O_2\left(đktc\right)}=5.\left(1,5.22,4\right)=168\left(lít\right)\)

Gọi \(\left\{{}\begin{matrix}n_{CO_2}=a\left(mol\right)\\n_{H_2O}=b\left(mol\right)\end{matrix}\right.\)
\(m_{giảm}=m_{CaCO_3}-m_{CO_2}-m_{H_2O}\)
=> 60 - 44a - 18b = 12
=> 44a + 18b = 48 (1)
\(a=n_{CO_2}=n_{CaCO_3}=\dfrac{60}{100}=0,6\left(mol\right)\)
=> b = 1,2 (mol)
Bảo toàn C: nC = 0,6 (mol)
Bảo toàn H: nH = 2,4 (mol)
=> m = 0,6.12 + 2,4.1 = 9,6 (g)
Bảo toàn O: \(n_{O_2}=\dfrac{2a+b}{2}=1,2\left(mol\right)\)
=> VO2 = 1,2.22,4 = 26,88 (l)
=> A

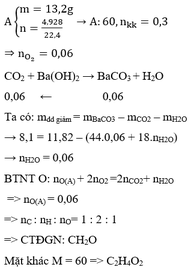
CH4 + 2O2 --> CO2 + 2H2O
a) Theo phản ứng: V O2=V CH4=4,48.2=8,96 lít
b) V CO2=V CH4=4,48 lít -> nCO2=4,48/22,4=0,2 mol
c) CO2 + Ba(OH)2 ---> BaCO3 + H2O
-> nCO2=nBaCO3=0,2 mol -> mBaCO3=0,2.197=39,4 gam
mH2O=0,2 .18=3,6 g