Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(1.a)QToxh:S^{+4}\rightarrow S^{+6}+2e|\times3\\ QTkhử:N^{+5}+3e\rightarrow N^{+2}|\times2\\ 3SO_2+2HNO_3+2H_2O\rightarrow2NO+3H_2SO_4\\ b)QToxh:\overset{0}{Fe}\rightarrow Fe^{3+}+3e|\times1\\ QTkhử:N^{+5}+1e\rightarrow N^{+4}|\times3\\ Fe+6HNO_3\rightarrow Fe\left(NO_3\right)_3+3NO_2+3H_2O\)

Gọi số mol Zn, Fe là a, b (mol)
=> 65a + 56b = 23,75 (1)
\(n_{H_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\)
PTHH: Zn + 2HCl --> ZnCl2 + H2
a--------------->a------->a
Fe + 2HCl --> FeCl2 + H2
b----------------->b----->b
=> a + b = 0,4 (2)
(1)(2) => a = 0,15 (mol); b = 0,25 (mol)
=> mZn = 0,15.65 = 9,75 (g); mFe = 0,25.56 = 14 (g)
\(\left\{{}\begin{matrix}\%m_{Zn}=\dfrac{9,75}{23,75}.100\%=41,05\%\\\%m_{Fe}=\dfrac{14}{23,75}.100\%=58,95\%\end{matrix}\right.\)
b) mZnCl2 = 0,15.136 = 20,4 (g)
mFeCl2 = 0,25.127 = 31,75 (g)
=> mmuối = 20,4 + 31,75 = 52,15 (g)

\(n_{H_2}=\dfrac{7,84}{22,4}=0,35mol\)
Gọi \(\left\{{}\begin{matrix}n_{Fe}=x\\n_{Zn}=y\end{matrix}\right.\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
x x ( mol )
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
y y ( mol )
Ta có:
\(\left\{{}\begin{matrix}56x+65y=21,4\\x+y=0,35\end{matrix}\right.\) \(\Leftrightarrow\left\{{}\begin{matrix}x=0,15\\y=0,2\end{matrix}\right.\)
\(\Rightarrow m_{Fe}=0,15.56=8,4g\)
\(\Rightarrow m_{Zn}=0,2.65=13g\)
\(\%m_{Fe}=\dfrac{8,4}{21,4}.100=39,25\%\)
\(\%m_{Zn}=100\%-39,25\%=60,75\%\)
\(m_{FeCl_2}=0,15.127=19,05g\)
\(m_{ZnCl_2}=0,2.136=27,2g\)

$PTHH:2KMnO_4+16HCl\to 2KCl+2MnCl_2+5Cl_2\uparrow+8H_2O(1)$
$2Fe+3Cl_2\xrightarrow{t^o}2FeCl_3(2)$
$n_{KMnO_4}=\dfrac{47,4}{158}=0,3(mol);n_{Fe}=\dfrac{16,8}{56}=0,3(mol)$
Theo PT: $n_{Cl_2(1)}=0,75(mol)\Rightarrow n_{Cl_2(2)}=0,75(mol)$
Lập tỉ lệ: $\dfrac{n_{Cl_2(2)}}{3}>\dfrac{n_{Fe}}{2}\Rightarrow Cl_2$ dư
$\Rightarrow n_{FeCl_3}=n_{Fe}=0,3(mol)$
$\Rightarrow m_{FeCl_3}=0,3.162,5=48,75(g)$

HD:
a)
FeS - 9e = Fe+3 + S+6
N+5 + 3e = N+2
------------------------------------
FeS + 3N+5 = Fe+3 + S+6 + 3N+2
FeS + 6HNO3 ---> Fe(NO3)3 + H2SO4 + 3NO + 2H2O
b)
Fe+3 + e = Fe+2
2I- -2e = I2
-------------------------
2Fe+3 + 2I- = 2Fe+2 + I2
2FeCl3 + 2KI ---> 2FeCl2 + I2 + 2KCl
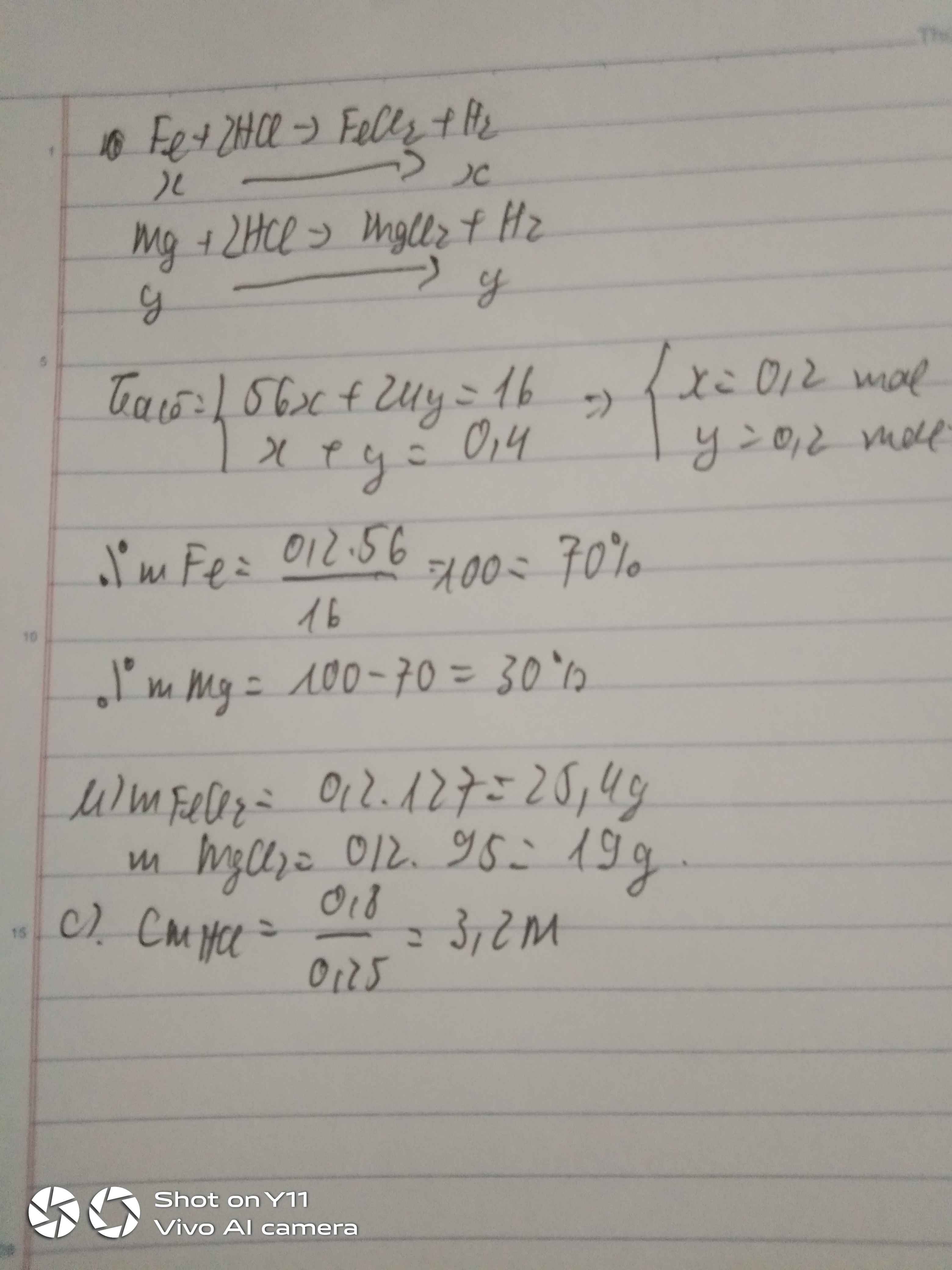
a)
$2Fe + 3Cl_2 \xrightarrow{t^o} 2FeCl_3$
b)
$n_{Fe} = \dfrac{28}{56} = 0,5(mol)$
$n_{Cl_2} = \dfrac{2,479}{24,79} = 0,1(mol)$
Ta thấy :
$n_{Fe} :2>n_{Cl_2}:3$ nên $Fe$ dư
$n_{FeCl_3} = \dfrac{2}{3}n_{Cl_2} = \dfrac{0,2}{3}(mol)$
$\Rightarrow m_{FeCl_3} = \dfrac{0,2}{3}.162,5 = 10,83(gam)$