Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a, PT: \(CuO+H_2\underrightarrow{t^o}Cu+H_2O\)
Ta có: \(n_{CuO}=\dfrac{3,2}{80}=0,04\left(mol\right)\)
Theo PT: \(n_{Cu}=n_{H_2O}=n_{CuO}=0,04\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}m_{Cu}=0,04.64=2,56\left(g\right)\\m_{H_2O}=0,04.18=0,72\left(g\right)\end{matrix}\right.\)
b, PT: \(Fe_3O_4+4H_2\underrightarrow{t^o}3Fe+4H_2O\)
Ta có: \(n_{Fe_3O_4}=\dfrac{10,8}{232}=\dfrac{27}{580}\left(mol\right)\)
\(n_{H_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
Xét tỉ lệ: \(\dfrac{\dfrac{27}{580}}{1}< \dfrac{0,2}{4}\), ta được H2 dư.
Theo PT: \(\left\{{}\begin{matrix}n_{H_2\left(pư\right)}=n_{H_2O}=4n_{Fe_3O_4}=\dfrac{27}{145}\left(mol\right)\\n_{Fe}=3n_{Fe_3O_4}=\dfrac{81}{580}\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow n_{H_2\left(dư\right)}=0,2-\dfrac{27}{145}=\dfrac{2}{145}\left(mol\right)\)
\(\Rightarrow m_{H_2\left(dư\right)}=\dfrac{2}{145}.2\approx0,0276\left(g\right)\)
\(m_{H_2O}=\dfrac{27}{145}.18\approx3,35\left(g\right)\)
\(m_{Fe}=\dfrac{81}{580}.56\approx7,82\left(g\right)\)
Bạn tham khảo nhé!

\(n_{Al}=\dfrac{5,4}{27}=0,2\left(mol\right)\\ n_{O_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\\a, PTHH:4Al+3O_2\rightarrow\left(t^o\right)2Al_2O_3\\ Vì:\dfrac{0,2}{4}< \dfrac{0,2}{3}\Rightarrow O_2dư\\ \Rightarrow n_{O_2\left(dư\right)}=0,2-\dfrac{3}{4}.0,2=0,05\left(mol\right)\\ \Rightarrow m_{O_2\left(dư\right)}=0,05.32=1,6\left(g\right)\\ b,n_{Al_2O_3}=\dfrac{n_{Al}}{2}=\dfrac{0,2}{2}=0,1\left(mol\right)\\ \Rightarrow m_{Al_2O_3}=102.0,1=10,2\left(g\right)\)
a, Ta có: \(n_{Al}=\dfrac{5,4}{27}=0,2\left(mol\right)\)
\(n_{O_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
PT: \(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
Xét tỉ lệ: \(\dfrac{0,2}{4}< \dfrac{0,2}{3}\), ta được O2 dư.
Theo PT: \(n_{O_2\left(pư\right)}=\dfrac{3}{4}n_{Al}=0,15\left(mol\right)\)
\(\Rightarrow n_{O_2\left(dư\right)}=0,05\left(mol\right)\)
\(\Rightarrow m_{O_2\left(dư\right)}=0,05.32=1,6\left(g\right)\)
b, Theo PT: \(n_{Al_2O_3}=\dfrac{1}{2}n_{Al}=0,1\left(mol\right)\)
\(\Rightarrow m_{Al_2O_3}=0,1.102=10,2\left(g\right)\)
Bạn tham khảo nhé!

\(a.n_{CuO}=\dfrac{40}{80}=0,5\left(mol\right)\\ n_{H_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\\ PTHH:CuO+H_2\underrightarrow{t^o}Cu+H_2O\\ Vì:\dfrac{0,15}{1}< \dfrac{0,5}{1}\\ \rightarrow CuOdư\\ n_{CuO\left(p.ứ\right)}=n_{Cu}=n_{H_2}=0,15\left(mol\right)\\ \rightarrow n_{CuO\left(dư\right)}=0,5-0,15=0,35\left(mol\right)\\ m_{CuO\left(DƯ\right)}=0,35.80=28\left(g\right)\\ b.m_{Cu}=0,35.64=22,4\left(g\right)\\ c.m_{hh_{rắn}}=m_{Cu}+m_{CuO\left(dư\right)}=22,4+28=50,4\left(g\right)\)

Nếu có thể thì lần sau bạn nên đăng tách từng bài ra nhé!
Bài 1:
PT: \(Mg+2HCl\rightarrow MgCl_2+H_2\)
Ta có: \(n_{Mg}=\dfrac{2,4}{24}=0,1\left(mol\right)\)
\(n_{HCl}=\dfrac{3,65}{36,5}=0,1\left(mol\right)\)
Xét tỉ lệ: \(\dfrac{0,1}{1}>\dfrac{0,1}{2}\) , ta được Mg dư.
Theo PT: \(n_{Mg\left(pư\right)}=n_{MgCl_2}=n_{H_2}=\dfrac{1}{2}n_{HCl}=0,05\left(mol\right)\)
\(\Rightarrow n_{Mg\left(dư\right)}=0,1-0,05=0,05\left(mol\right)\)
\(\Rightarrow m_{Mg\left(dư\right)}=0,05.24=1,2\left(g\right)\)
\(m_{MgCl_2}=0,05.95=4,75\left(g\right)\)
\(V_{H_2}=0,05.22,4=1,12\left(l\right)\)
Bài 2:
PT: \(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
Ta có: \(n_{Al}=\dfrac{5,4}{27}=0,2\left(mol\right)\)
\(n_{H_2SO_4}=\dfrac{14,7}{98}=0,15\left(mol\right)\)
Xét tỉ lệ: \(\dfrac{0,2}{2}>\dfrac{0,15}{3}\) , ta được Al dư.
Theo PT: \(\left\{{}\begin{matrix}n_{Al\left(pư\right)}=\dfrac{2}{3}n_{H_2SO_4}=0,1\left(mol\right)\\n_{Al_2\left(SO_4\right)_3}=\dfrac{1}{3}n_{H_2SO_4}=0,05\left(mol\right)\\n_{H_2}=n_{H_2SO_4}=0,15\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow n_{Al\left(dư\right)}=0,2-0,1=0,1\left(mol\right)\)
\(\Rightarrow m_{Al\left(dư\right)}=0,1.27=2,7\left(g\right)\)
\(m_{Al_2\left(SO_4\right)_3}=0,05.342=17,1\left(g\right)\)
\(V_{H_2}=0,15.22,4=3,36\left(l\right)\)
Bài 3:
PT: \(2M+6HCl\rightarrow2MCl_3+3H_2\)
Ta có: \(n_{H_2}=\dfrac{4,704}{22,4}=0,21\left(mol\right)\)
Theo PT: \(n_M=\dfrac{2}{3}n_{H_2}=0,14\left(mol\right)\)
\(\Rightarrow M_M=\dfrac{3,78}{0,14}=27\left(g/mol\right)\)
Vậy: M là nhôm (Al).
Bài 4:
PT: \(2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\)
Theo PT: \(n_{O_2}=\dfrac{1}{2}n_{KMnO_4}=0,2\left(mol\right)\)
PT: \(4P+5O_2\underrightarrow{t^o}2P_2O_5\)
Ta có: \(n_P=\dfrac{6,2}{31}=0,2\left(mol\right)\)
Xét tỉ lệ: \(\dfrac{0,2}{4}>\dfrac{0,2}{5}\) , ta được P dư.
Theo PT: \(n_{P_2O_5}=\dfrac{2}{5}n_{O_2}=0,08\left(mol\right)\)
\(\Rightarrow m_{P_2O_5}=0,08.142=11,36\left(g\right)\)
Bạn tham khảo nhé!

a, Theo gt ta có: $n_{H_2}=0,15(mol);n_{O_2}=0,05(mol)$
$2H_2+O_2\rightarrow 2H_2O$
Sau phản ứng $H_2$ còn dư. Và dư 0,05.22,4=1,12(l)
b, Ta có: $n_{H_2O}=2.n_{O_2}=0,1(mol)\Rightarrow m_{H_2O}=1,8(g)$

\(a.n_{Fe_2O_3}=\dfrac{24}{160}=0,15\left(mol\right)\\ n_{H_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\\ Fe_2O_3+3H_2\underrightarrow{to}2Fe+3H_2O\\ Vì:\dfrac{0,3}{3}< \dfrac{0,15}{1}\\ \rightarrow Fe_2O_3dư\\ n_{Fe_2O_3\left(dư\right)}=0,15-\dfrac{0,3}{3}=0,05\left(mol\right)\\ m_{Fe_2O_3\left(dư\right)}=0,05.160=8\left(g\right)\\ b.n_{Fe}=\dfrac{0,3}{3}.2=0,2\left(mol\right)\\ m_{Fe}=0,2.56=11,2\left(g\right)\\ c.m_{rắn}=m_{Fe}+m_{Fe_2O_3\left(dư\right)}=11,2+8=19,2\left(g\right)\)

Anh đoán là đkc chứ không phải đktc nhưng em cứ phản hồi nhé!

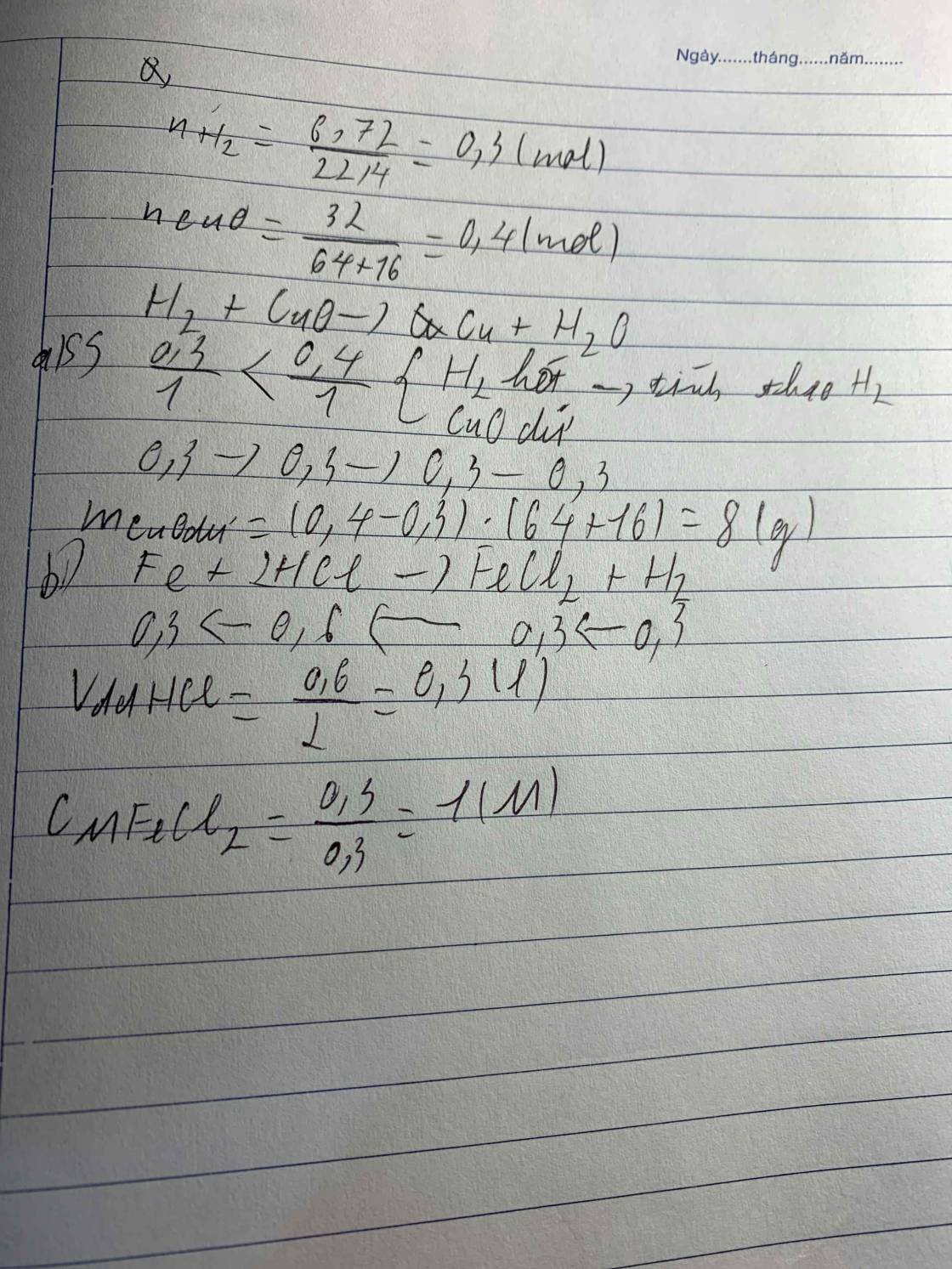
\(n_{H_2}=\dfrac{V_{H_2}}{22,4}=\dfrac{4,48}{22,4}=0,2mol\)
\(n_{Fe_2O_3}=\dfrac{m_{Fe_2O_3}}{M_{Fe_2O_3}}=\dfrac{23,2}{160}=0,145mol\)
\(Fe_2O_3+3H_2\rightarrow\left(t^o\right)2Fe+3H_2O\)
0,145 > 0,2 ( mol )
1/15 0,2 2/15 ( mol 0
Chất còn dư là \(Fe_2O_3\)
\(m_{Fe_2O_3\left(du\right)}=n_{Fe_2O_3\left(du\right)}.M_{Fe_2O_3}=\left(0,145-\dfrac{1}{15}\right).160=12,53g\)
\(m_{Fe}=n_{Fe}.M_{Fe}=\dfrac{2}{15}.56=7,4666g\)