Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(a)n_{CH_3COOH} = 0,2.2 = 0,4(mol)\\ Mg + 2CH_3COOH \to (CH_3COO)_2Mg + H_2\\ n_{Mg} = \dfrac{1}{2}n_{CH_3COOH} = 0,2(mol)\\ m_{Mg} = 0,2.24 = 4,8(gam)\\ b)\\ CH_3COOH + C_2H_5OH \buildrel{{H_2SO_4}}\over\rightleftharpoons CH_3COOC_2H_5 + H_2O\\ n_{CH_3COOH\ pư} = n_{este} = \dfrac{24,64}{88} = 0,28(mol)\\ H = \dfrac{0,28}{0,4}.100\% = 70\%\)

\(m_{CH_3COOH}=150.30\%=45\left(g\right)\\ n_{CH_3COOH}=\dfrac{45}{60}=0,75\left(mol\right)\)
PTHH: CH3COOH + C2H5OH \(\xrightarrow[t^o]{H_2SO_4đặc}\) CH3COOC2H5 + H2O
0,75 ----------> 0,75 ------------------> 0,75
\(V_{C_2H_5OH}=\dfrac{46.0,75}{0,8}=43,125\left(ml\right)\\ m_{CH_3COOC_2H_5}=0,75.45\%.88=29,7\left(g\right)\)

a.b.\(n_{Mg}=\dfrac{4,8}{24}=0,2mol\)
\(2Mg+2CH_3COOH\rightarrow2\left(CH_3COO\right)_2Mg+H_2\)
0,2 0,2 0,2 ( mol )
\(C_{M_{CH_3COOH}}=\dfrac{0,2}{0,2}=1M\)
\(m_{\left(CH_3COO\right)_2Mg}=0,2.142=28,4g\)
c.Sửa đề: thu được 9,2g este
\(n_{CH_3COOC_2H_5}=\dfrac{9,2}{88}=0,1mol\)
\(CH_3COOH+C_2H_5OH\rightarrow CH_3COOC_2H_5+H_2O\)
Thực tế: 0,2 0,1 ( mol )
Lý thuyết: 0,1 0,1 ( mol )
\(H=\dfrac{0,1}{0,2}.100=50\%\)

\(n_{BaCO_3}=\dfrac{19,7}{197}=0,1\left(mol\right)\\ a,PTHH:BaCO_3+2CH_3COOH\rightarrow\left(CH_3COO\right)_2Ba+CO_2+H_2O\\ n_{CO_2}=n_{\left(CH_3COO\right)_2Ba}=n_{BaCO_3}=0,1\left(mol\right)\\ b,V_{CO_2\left(đktc\right)}=0,1.22,4=2,24\left(l\right)\\ c,m_{\left(CH_3COO\right)_2Ba}=255.0,1=25,5\left(g\right)\\ d,C_2H_5OH+O_2\rightarrow\left(men.giấm\right)CH_3COOH+H_2O\\ n_{CH_3COOH}=0,1.2=0,2\left(mol\right);n_{C_2H_5OH\left(LT\right)}=n_{CH_3COOH}=0,2\left(mol\right)\\ n_{C_2H_5OH\left(TT\right)}=0,2.75\%=0,15\left(mol\right)\\ m_{C_2H_5OH\left(TT\right)}=0,15.46=6,9\left(g\right)\)

a, \(n_{CH_3COOH}=0,2.1=0,2\left(mol\right)\)
PT: \(Mg+2CH_3COOH\rightarrow\left(CH_3COO\right)_2Mg+H_2\)
Theo PT: \(n_{Mg}=n_{H_2}=\dfrac{1}{2}n_{CH_3COOH}=0,1\left(mol\right)\)
\(\Rightarrow m=m_{Mg}=0,1.24=2,4\left(g\right)\)
\(V=V_{H_2}=0,1.22,4=2,24\left(l\right)\)
b, \(C_2H_5OH+O_2\underrightarrow{^{mengiam}}CH_3COOH+H_2O\)
Theo PT: \(n_{C_2H_5OH}=n_{CH_3COOH}=0,2\left(mol\right)\)
\(\Rightarrow m_{C_2H_5OH}=0,2.46=9,2\left(g\right)\)
\(\Rightarrow V_{ddC_2H_5OH}=\dfrac{9,2}{0,8}=11,5\left(ml\right)\)

a/ \(n_{H_2}=\dfrac{7,437}{24,79}=0,3\left(mol\right)\)
PTHH: Mg + 2HCl → MgCl2 + H2
Mol: 0,3 0,6 0,3 0,3
\(m_{Mg}=0,3.24=7,2\left(g\right)\)
b/ \(m_{MgCl_2}=0,3.95=28,5\left(g\right)\)
c/ \(m_{HCl}=0,3.36,5=10,95\left(g\right)\)
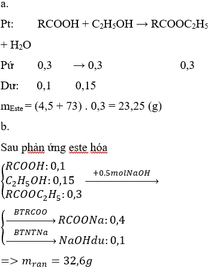
a) \(n_{CH_3COOH}=\dfrac{30}{60}=0,5\left(mol\right)\)
PTHH: CH3COOH + C2H5OH --H2SO4(đ),to--> CH3COOC2H5 + H2O
0,5------->0,5------------------------>0,5
=> mC2H5OH = 0,5.46 = 23 (g)
b) mCH3COOC2H5 = 0,5.88 = 44 (g)