Cho 32,5g Zn tác dụng vs dung dịch có chứa 29,2g HCl. Tính thể tích khí H2( đktc) thu được
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


PTHH: \(Zn+2HCl\rightarrow ZnCl_2+H_2\uparrow\)
Ta có: \(n_{Zn}=\dfrac{32,5}{65}=0,5\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}n_{HCl}=1\left(mol\right)\\n_{H_2}=0,5\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}m_{ddHCl}=\dfrac{1\cdot36,5}{20\%}=182,5\left(g\right)\\V_{H_2}=0,5\cdot22,4=11,2\left(l\right)\end{matrix}\right.\)

PTHH: \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
+\(n_{Zn}=\dfrac{32,5}{65}=0,5\left(mol\right)\)
+\(nH_2=n_{Zn}=0,5\left(mol\right)\)
+\(n_{HCl}=2n_{Zn}=1\left(mol\right)\)
+\(V_{H2}=0,5.22,4=11,2\left(lit\right)\)
\(m_{HCl}=1.36,5=36,5\left(gam\right)\)
\(n_{Zn}=\dfrac{m}{M}=\dfrac{32,5}{65}=0,5\left(mol\right)\)
\(Zn\) \(+\) \(2\)\(HCl\) → \(ZnCl_2\) \(+\) \(H_2\)
\(0,5\) \(mol\) → \(1\) \(mol\) → \(0,5\)\(mol\) → \(0,5\) \(mol\)
\(V_{H_2}=n.22,4=0,5.22,4=11,2\left(l\right)\)
\(m_{HCl}=n.M=1.36,5=36,4\left(g\right)\)

\(n_{Zn}=\dfrac{8,125}{65}=0,125mol\)
\(n_{HCl}=\dfrac{18,25}{36,5}=0,5mol\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
Xét: \(\dfrac{0,125}{1}\) < \(\dfrac{0,5}{2}\) ( mol )
0,125 0,125 ( mol )
\(V_{H_2}=0,125.22,4=2,8l\)

\(n_{Zn}=\dfrac{13}{65}=0,2\left(mol\right)\); \(n_{HCl}=\dfrac{14,6}{36,5}=0,4\left(mol\right)\)
PTHH: Zn + 2HCl --> ZnCl2 + H2
Xét tỉ lệ: \(\dfrac{0,2}{1}=\dfrac{0,4}{2}\) => pư vừa đủ
PTHH: Zn + 2HCl --> ZnCl2 + H2
0,2----------------->0,2
=> VH2 = 0,2.22,4 = 4,48 (l)

nZn = 0.65 / 65 = 0.01 (mol)
Zn + 2HCl => ZnCl2 + H2
0.01..................0.01......0.01
mZnCl2 = 0.01 * 136 = 1.36 (g)
VH2 = 0.01 * 22.4 = 0.224 (l)

\(n_{Zn}=\dfrac{6,5}{65}=0,1mol\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
0,1 0,2 0,1 0,1
\(V_{H_2}=0,1\cdot22,4=2,24l\)

\(13,n_{Fe}=\dfrac{16,8}{56}=0,3\left(mol\right)\\ PTHH:Fe+2HCl\rightarrow FeCl_2+H_2\\ .....0,3.....0,6......0,3......0,3\left(mol\right)\\ V_{H_2\left(đktc\right)}=0,3\cdot22,4=6,72\left(l\right)\\ 14,n_{CaCO_3}=\dfrac{25}{40+12+16\cdot3}=0,25\left(mol\right)\\ PTHH:CaCO_3+2HCl\rightarrow CaCl_2+H_2O+CO_2\\ .....0,25.....0,5......0,25......0,25......0,25\left(mol\right)\\ V_{CO_2\left(đktc\right)}=0,25\cdot22,4=5,6\left(l\right)\)
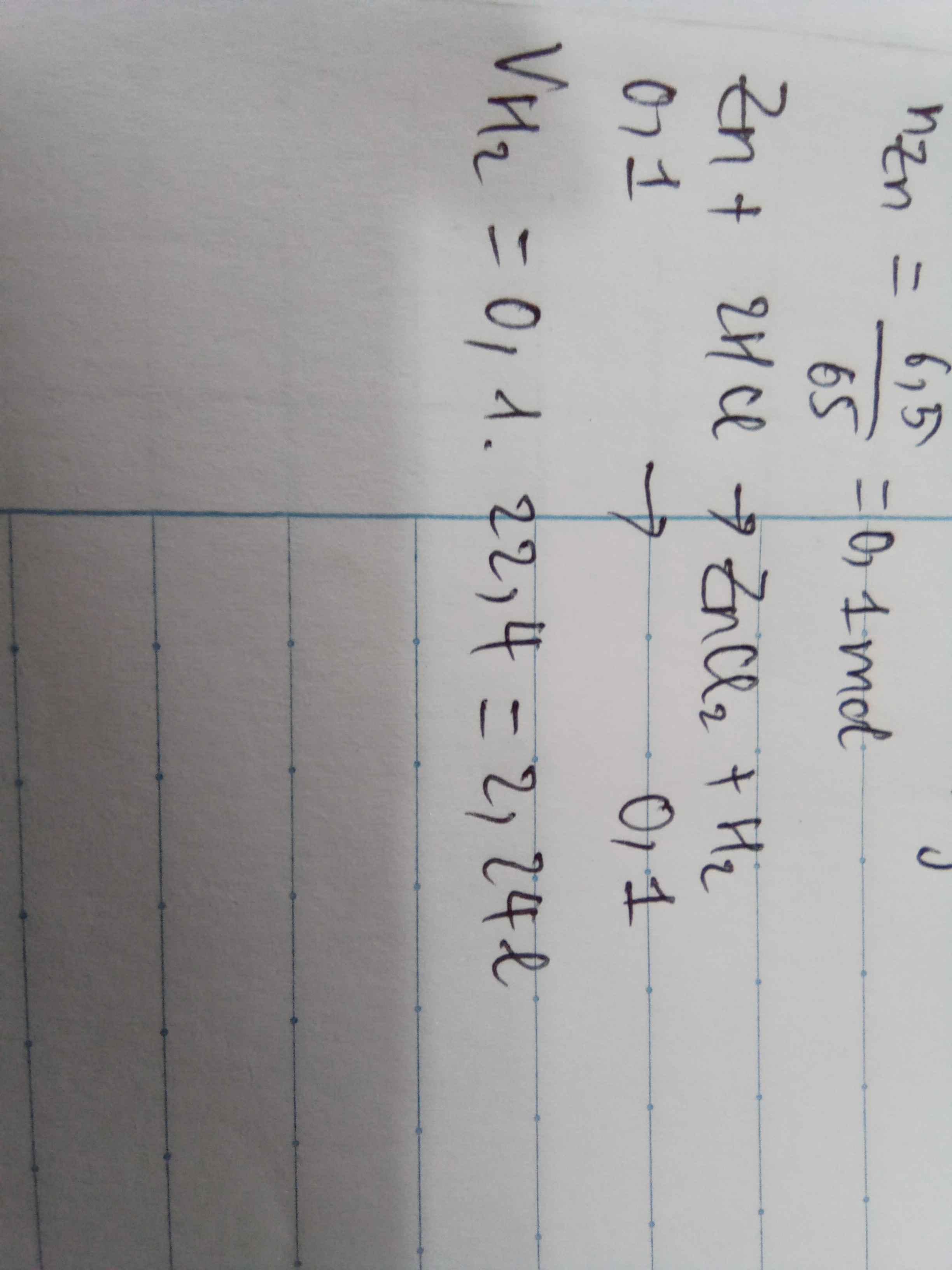
\(n_{Zn}=\dfrac{32.5}{65}=0.5\left(mol\right)\)
\(n_{HCl}=\dfrac{29.2}{36.5}=0.8\left(mol\right)\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
\(1.........2\)
\(0.5......0.8\)
\(LTL:\dfrac{0.5}{1}>\dfrac{0.8}{2}\Rightarrow Zndư\)
\(V_{H_2}=0.4\cdot22.4=8.96\left(l\right)\)