Tính khối lượng phân tử sao: a.H2SO4 b.Al2(SO4)3 c.Ca(OH)2 d.NaHCO3
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


\(PTK_{Ca\left(OH\right)_2}=NTK_{Ca}+2.\left[NTK_O+NTK_H\right]=40+2.\left(16+1\right)=74\left(đ.v.C\right)\\ PTK_{Fe\left(OH\right)_3}=NTK_{Fe}+3.\left[NTK_O+NTK_H\right]=56+3.\left(16+1\right)=107\left(đ.v.C\right)\\ PTK_{KNO_3}=NTK_K+NTK_N+3.NTK_O=39+14+3.16=101\left(đ.v.C\right)\\ PTK_{Fe_2O_3}=2.NTK_{Fe}+3.NTK_O=2.56+3.16=160\left(đ.v.C\right)\)
\(PTK_{N_2O_5}=2.NTK_N+5.NTK_O=2.14+5.16=108\left(đ.v.C\right)\\ PTK_{MgSO_4}=NTK_{Mg}+NTK_S+4.NTK_O=24+32+4.16=120\left(đ.v.C\right)\\ PTK_{Al_2\left(SO_4\right)_3}=2.NTK_{Al}+3.\left[NTK_S+3.4.NTK_O\right]\\ =2.27+3.\left(32+3.4.16\right)=342\left(đ.v.C\right)\\ PTK_{BaCO_3}=NTK_{Ba}+NTK_C+3.NTK_O=137+12+3.16=197\left(đ.v.C\right)\)

Dạng này em tính phân tử khối, nguyên tử khối rồi nhân với 0,16605.10-23 (g)
Trả lời:
\(a)\)
\(m_C=1,6605.10^{-24}.12=1,9926.10^{-23}\left(g\right)\)
\(m_{Cl}=1,6605.10^{-24}.35,5=5,894775.10^{-23}\left(g\right)\)
\(m_{KOH}=1,6605.10^{-24}.\left(39+16+1\right)=9,2988.10^{-23}\left(g\right)\)
\(m_{H2SO4}=1,6605.10^{-24}.\left(2+32+4.16\right)=1,62729.10^{-22}\left(g\right)\)
\(m_{Fe2\left(CO3\right)3}=1,6605.10^{-24}.\left(2.56+\left(12+3.16\right).3\right)=4,84866.10^{-22}\left(g\right)\)
+) Đơn chất: \(C,Cl.\)
+) Hợp chất: \(KOH,H_2SO_4,Fe_2\left(CO_3\right)_3.\)
\(b)\)
\(m_{BaSO4}=1,6605.10^{-24}.\left(137+32+4.16\right)=3,868965.10^{-22}\left(g\right)\)
\(m_{O2}=1,6605.10^{-24}.\left(2.16\right)=5,3136.10^{-23}\left(g\right)\)
\(m_{Ca\left(OH\right)2}=1,6605.10^{-24}.\left(40+\left(16+1\right).2\right)=1,22877.10^{-22}\left(g\right)\)
\(m_{Fe}=1,6605.10^{-24}.56=9,2988.10^{-23}\left(g\right)\)
+) Đơn chất: \(O_2,Fe.\)
+) Hợp chất: \(BaSO_4,Ca\left(OH\right)_2.\)
\(c)\)
\(m_{HCl}=1,6605.10^{-24}.\left(1+35,5\right)=6,060825.10^{-23}\left(g\right)\)
\(m_{NO}=1,6605.10^{-24}.\left(14+16\right)=4,9815.10^{-23}\left(g\right)\)
\(m_{Br2}=1,6605.10^{-24}.\left(2.80\right)=2,6568.10^{-22}\left(g\right)\)
\(m_K=1,6605.10^{-24}.39=6,47595.10^{-23}\left(g\right)\)
\(m_{NH3}=1,6605.10^{-24}.\left(14+3.1\right)=2,82285.10^{-23}\left(g\right)\)
+) Đơn chất: \(Br_2,K.\)
+) Hợp chất: \(HCl,NO,NH_3.\)
\(d)\)
\(m_{C6H5OH}=1,6605.10^{-24}.\left(12.6+5.1+16+1\right)=1,56087.10^{-22}\left(g\right)\)\(m_{CH4}=1,6605.10^{-24}.\left(12+4.1\right)=2,6568.10^{-23}\left(g\right)\)
\(m_{O3}=1,6605.10^{-24}.\left(3.16\right)=7,9704.10^{-23}\left(g\right)\)
\(m_{BaO}=1,6605.10^{-24}.\left(137+16\right)=2,540565.10^{-22}\left(g\right)\)
+) Đơn chất: \(O_3\)
+) Hợp chất: \(C_6H_5OH,CH_4,BaO.\)

\(PTK_{Mg\left(OH\right)_2}=24+\left(16+1\right).2=58\left(đvC\right)\)
\(PTK_{Ca\left(H_2PO_4\right)_2}=40+\left(1.2+31+16.4\right).2=234\left(đvC\right)\)
\(PTK_{Ba_3\left(PO_4\right)_2}=137.3+\left(31+16.4\right).2=601\left(đvC\right)\)
\(PTK_{Al_2\left(SO_4\right)_3}=27.2+\left(32+16.4\right).3=342\left(đvC\right)\)
\(PTK_{Ca\left(HCO_3\right)_2}=40+\left(1+12+16.3\right).2=162\left(đvC\right)\)
\(PTK_{Fe\left(NO_3\right)_2}=56+\left(14+16.3\right).2=180\left(đvC\right)\)
\(PTK_{Mg\left(OH\right)_2}=24+\left(16+1\right)\cdot2=58\left(đvC\right)\\ PTK_{Ca\left(H_2PO_4\right)_2}=40+\left(2+31+16\cdot4\right)\cdot2=234\left(đvC\right)\\ PTK_{Ba_3\left(PO_4\right)_2}=137\cdot3+\left(31+16\cdot4\right)\cdot2=601\left(đvC\right)\\ PTK_{Al_2\left(SO_4\right)_3}=27\cdot2+\left(32+16\cdot4\right)\cdot3=342\left(đvC\right)\\ PTK_{Ca\left(HCO_3\right)_2}=40+\left(1+12+16\cdot3\right)\cdot2=162\left(đvC\right)\\ PTK_{Fe\left(NO_3\right)_2}=56+\left(14+16\cdot3\right)\cdot2=180\left(đvC\right)\)

1.
\(PTK_{CuSO_4}=64+32+16.4=160\left(đvC\right)\)
\(PTK_{5CaCO_3}=5\left(40+12+16.3\right)=500\left(đvC\right)\)
\(PTK_{Ca\left(OH\right)_2}=40+\left(16+1\right).2=74\left(đvC\right)\)
2.
Theo đề, ta có:
\(d_{\dfrac{X}{Mg}}=\dfrac{M_X}{M_{Mg}}=\dfrac{M_X}{24}=\dfrac{4}{3}\left(lần\right)\)
=> MX = 32(g)
Vậy X là lưu huỳnh (S)
3.
Ta có: \(PTK_{Al_x\left(SO_4\right)_3}=27.x+\left(32+16.4\right).3=342\left(đvC\right)\)
=> x = 2
Bài 1.Phân tử khối các chất:
\(CuSO_4\)\(\Rightarrow64+32+4\cdot16=160\left(đvC\right)\)
\(CaCO_3\Rightarrow40+12+3\cdot16=100\left(đvC\right)\)
\(Ca\left(OH\right)_2\Rightarrow40+16\cdot2+2=74\left(đvC\right)\)
Bài 2.Theo bài: \(\overline{M_X}=\dfrac{4}{3}\overline{M_{Mg}}=\dfrac{4}{3}\cdot24=32\left(đvC\right)\)
Vậy X là lưu huỳnh.KHHH: S.
Bài 3. \(Al_x\left(SO_4\right)_3\) \(\Rightarrow27x+3\cdot\left(32+4\cdot16\right)=342\Leftrightarrow x=2\)
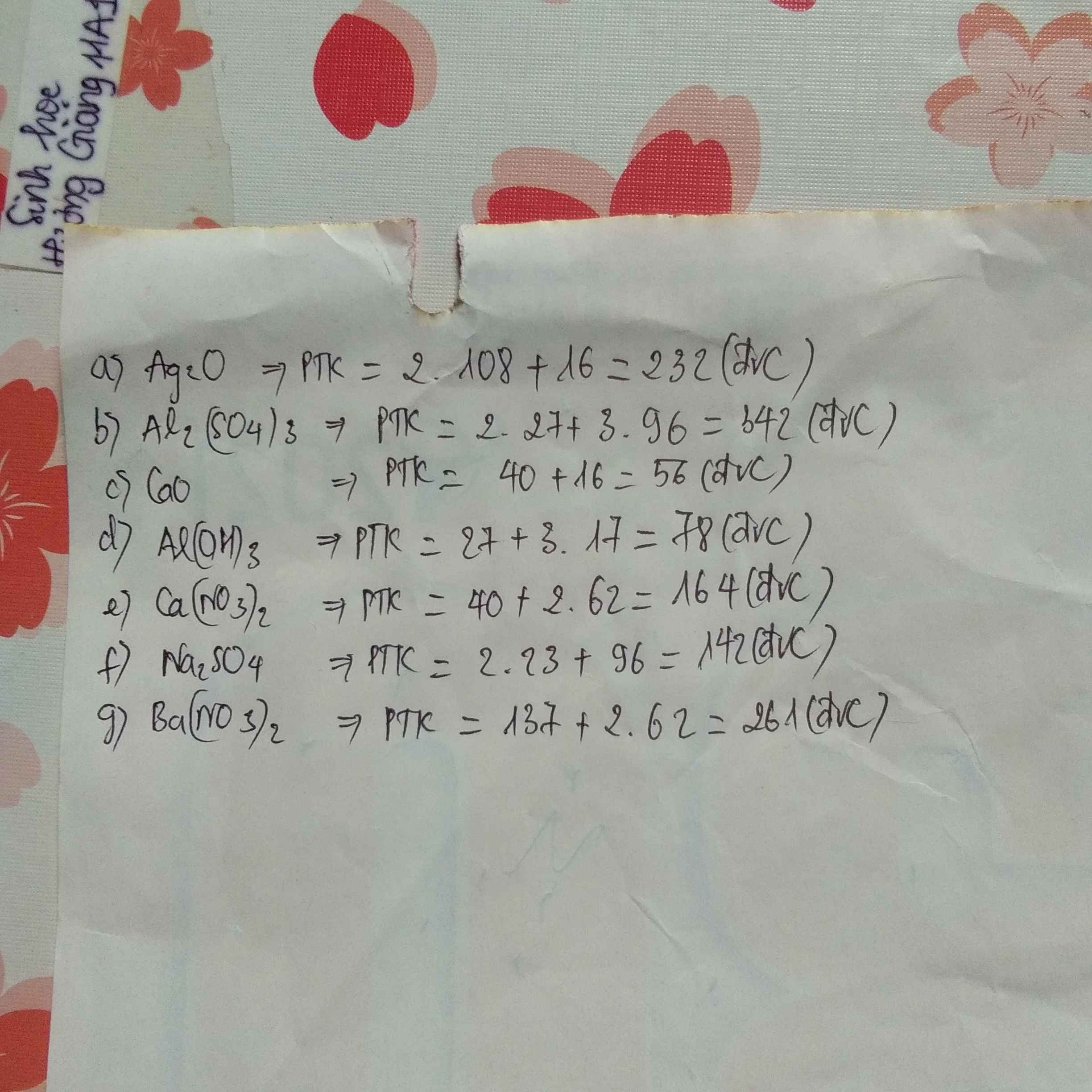
\(a)\) PTK \(H_2SO_4\) : \(1.2+32+16.4=98\left(đvC\right)\)
\(b)\) PTK \(Al_2\left(SO_4\right)_3\) : \(27.2+\left(32+16.4\right).3=342\left(đvC\right)\)
\(c)\) PTK \(Ca\left(OH\right)_2\) : \(40+\left(16+1\right).2=74\left(đvC\right)\)
\(d)\) PTK \(NaHCO_3\) : \(23+1+12+16.3=84\left(đvC\right)\)
đơn vị amu nhé