
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


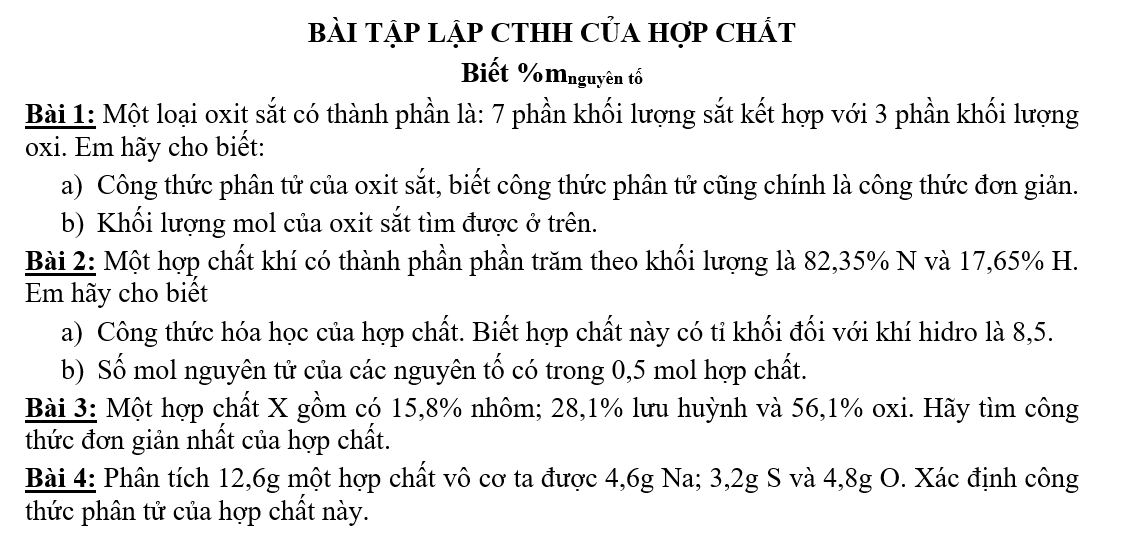
B4:
\(CTTQ:Na_xS_yO_z\left(x,y,z:nguy\text{ê}n,d\text{ươ}ng\right)\\ n_{Na}=\dfrac{4.6}{23}=0,2\left(mol\right);n_S=\dfrac{3,2}{32}=0,1\left(mol\right);n_O=\dfrac{4,8}{16}=0,3\left(mol\right)\\ x:y:z=0,2:0,1:0,3=2:1:3\\ \Rightarrow x=2;y=1;z=3\\ \Rightarrow CTHH:Na_2SO_3\)

1. Because of studying hard, I passed the exam
2. Because of Hoa's richness, she could buy that house
3. Because of bad grades, she failed the University entrance exam
4. Because of the accident, I was late
5. Because of the terrible traffic, we didn't arrive until 6 o'clock

Bài 1:
a. \(R=p\dfrac{l}{S}=1,10.10^{-6}\dfrac{30}{0,3\cdot10^{-6}}=110\Omega\)
b. \(I=U:R=220:110=2A\)
Bài 2:
a. \(R=R1+R2=30+50=80\Omega\)
b. \(I=I1=I2=0,25A\left(R1ntR2\right)\)
\(\left\{{}\begin{matrix}U1=I1\cdot R1=0,25\cdot30=7,5V\\U2=I2\cdot R2=0,25\cdot50=12,5V\\U=IR=0,25\cdot80=20V\end{matrix}\right.\)
Câu 1.
a)\(R=\rho\cdot\dfrac{l}{S}=1,1\cdot10^{-6}\cdot\dfrac{30}{0,3\cdot10^{-6}}=110\Omega\)
b)\(I=\dfrac{U}{R}=\dfrac{220}{110}=2A\)
Câu 2.
a)\(R_{AB}=R_1+R_2=30+50=80\Omega\)
b)\(I_1=I_2=I_A=0,25A\)
\(U_1=R_1\cdot I_1=30\cdot0,25=7,5V\)
\(U_2=R_2\cdot I_2=50\cdot0,25=12,5V\)
\(U_{AB}=U_1+U_2=7,5+12,5=20V\)
Câu 3.
a)\(R_{AB}=\dfrac{R_1\cdot R_2}{R_1+R_2}=\dfrac{600\cdot900}{600+900}=360\Omega\)
b)\(U_1=U_2=U_m=220V\)
\(I_1=\dfrac{U_1}{R_1}=\dfrac{220}{600}=\dfrac{11}{30}A\)
\(I_2=\dfrac{U_2}{R_2}=\dfrac{220}{900}=\dfrac{11}{45}A\)
\(I_m=I_1+I_2=\dfrac{11}{30}+\dfrac{11}{45}=\dfrac{11}{18}A\)

1. In spite of being a poor student, he studied very well
2. Despite the bad weather, she went to school on time
3. Despite having a physical handicap, she has become a successful woman
4. In spite of having not finished the paper, he went to sleep
5. Despite having a lot of noise in the city, I prefer living there
1. In spite of being a poor student, he studied very well
2. Despite the fact that the weather was bad, she went to school on time.



Câu 10:
\(a,Fe_2O_3+3CO\rightarrow\left(t^o\right)2Fe+3CO_2\\ b,2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\\ c,2Na+H_2SO_4\rightarrow Na_2SO_4+H_2\\ d,2KOH+H_2SO_4\rightarrow K_2SO_4+2H_2O\\ Fe\left(OH\right)_2+2HCl\rightarrow FeCl_2+H_2O\\ Fe_2\left(SO_4\right)_3+BaCl_2\rightarrow FeCl_2+BaSO_4\left(PTHH.Sai\right)\\ 2Al+3CuSO_4\rightarrow Al_2\left(SO_4\right)_3+3Cu\\ 2Al+3MgO\rightarrow\left(t^o\right)Al_2O_3+3Mg\\ 2Al+3Cl_2\rightarrow\left(t^o\right)2AlCl_3\)
Câu 8,9 đã làm!
Câu 7:
\(\text{Đ}\text{ặt}:N_xO_y\left(x,y:nguy\text{ê}n,d\text{ươ}ng\right)\\ V\text{ì}:\dfrac{m_N}{m_O}=\dfrac{7}{20}\\ \Leftrightarrow\dfrac{14x}{16y}=\dfrac{7}{20}\\ \Rightarrow\dfrac{x}{y}=\dfrac{7.16}{20.14}=\dfrac{2}{5}\\ \Rightarrow x=2;y=5\\ \Rightarrow CTHH:N_2O_5\)

a) \(d\dfrac{CH_4}{kk}=\dfrac{M_{CH_4}}{M_{kk}}=\dfrac{16}{29}< 1\)
⇒ \(CH_4\) nhẹ hơn kk
b) \(d\dfrac{N_2O}{kk}=\dfrac{M_{N_2O}}{M_{kk}}=\dfrac{44}{29}=1,52>1\)
⇒ \(N_2O\) nặng hơn kk
Câu b hỏi khí F2. Có hỏi không khí đâu??

\(15\left(\dfrac{m.}{s}\right)=54\left(\dfrac{km}{h}\right)\)
\(\Rightarrow v_{tb}=\dfrac{s}{\dfrac{\dfrac{1}{2}s}{v'}+\dfrac{\dfrac{1}{2}s}{v''}}=\dfrac{s}{\dfrac{s}{36}+\dfrac{s}{108}}=\dfrac{s}{\dfrac{4s}{108}}=\dfrac{108}{4}=27\left(\dfrac{km}{h}\right)\)
\(TBCv_1-v_2=\left(18+54\right):2=36\left(\dfrac{km}{h}\right)\)
\(\Rightarrow v_{tb}< TBCv_1-v_2\left(27< 36\right)\)
Vậy ta đc đpcm.








 nhiều!
nhiều!