Trộn 100ml dung dịch axit HCl 0,01M với 200ml dung dịch NaOH 0,5M thu được dung dịch A sau phản ứng.Viết phương trình phân tử và ion rút gọn của phản ứng và tính pH của dung dịch sau phản ứng.
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


\(2KOH+H_2SO_4->K_2SO_4+2H_2O\)
\(OH^-+H^+->H_2O\)
\(n_{H_2SO_4}=0,05.0,2=0,01\left(mol\right);n_{KOH}=0,2.0,1=0,02\left(mol\right)\)
PTHH: \(2KOH+H_2SO_4->K_2SO_4+2H_2O\)
_____0,02------->0,01
=> KOH, H2SO4 phản ứng vừa đủ, tạo ra dd K2SO4
=> pH = 7

\(n_{H^+}=0.2\cdot0.01\cdot2=0.004\left(mol\right)\)
\(n_{OH^-}=0.1\cdot0.01=0.001\left(mol\right)\)
\(H^++OH^-\rightarrow H_2O\)
\(0.001.....0.001\)
\(n_{H^+\left(dư\right)}=0.004-0.001=0.003\left(mol\right)\)
\(pH=-log\left(H^+\right)=-log\left(\dfrac{0.003}{0.2+0.1}\right)=2\)
Có: \(n_{H^+}=2n_{H_2SO_4}=2.0,2.0,01=0,004\left(mol\right)\)
\(n_{OH^-}=0,1.0,01=0,001\left(mol\right)\)
PT ion: \(H^++OH^-\rightarrow H_2O\)
____0,004___0,001 (mol)
\(\Rightarrow n_{H^+\left(dư\right)}=0,003\left(mol\right)\)\\(\Rightarrow\left[H^+\right]=\dfrac{0,003}{0,3}=0,01\)
\(\Rightarrow pH=2\)
Bạn tham khảo nhé!

a) PT phân tử: \(CaCO_3+2HCl\rightarrow CaCl_2+H_2O+CO_2\uparrow\)
PT ion: \(CaCO_3+2H^+\rightarrow Ca^{2+}+H_2O+CO_2\uparrow\)
b) Ta có: \(\left\{{}\begin{matrix}n_{CaCO_3}=\dfrac{10}{100}=0,1\left(mol\right)\\n_{HCl}=\dfrac{43,8\cdot20\%}{36,5}=0,24\left(mol\right)\end{matrix}\right.\)
Xét tỉ lệ: \(\dfrac{0,1}{1}< \dfrac{0,24}{2}\) \(\Rightarrow\) HCl dư, tính theo CaCO3
\(\Rightarrow\left\{{}\begin{matrix}n_{CaCl_2}=0,1\left(mol\right)=n_{CO_2}\\n_{HCl\left(dư\right)}=0,04\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}m_{CaCl_2}=0,1\cdot111=11,1\left(g\right)\\m_{CO_2}=0,1\cdot44=4,4\left(g\right)\\m_{HCl\left(dư\right)}=0,04\cdot36,5=1,46\left(g\right)\end{matrix}\right.\)
Mặt khác: \(m_{dd}=m_{CaCO_3}+m_{ddHCl}-m_{CO_2}=49,4\left(g\right)\)
\(\Rightarrow\left\{{}\begin{matrix}C\%_{CaCl_2}=\dfrac{11,1}{49,4}\cdot100\%\approx22,47\%\\C\%_{HCl\left(dư\right)}=\dfrac{1,46}{49,4}\cdot100\%\approx2,96\%\end{matrix}\right.\)

\(H^++OH^-\rightarrow H_2O\\ n_{H^+}=0,05\left(mol\right);n_{OH^-}=0,07\left(mol\right)\\ Lậptỉlệ:\dfrac{0,05}{1}< \dfrac{0,07}{1}\\ \Rightarrow OH^-dư\\ \left[OH^-_{dư}\right]=\dfrac{0,07-0,05}{0,2}=0,1M\\ \Rightarrow pOH=-log\left(0,1\right)=1\\ \Rightarrow pH=14-1=13\)

$n_{Ba^{2+}} = 0,1.0,5 = 0,05 < n_{SO_4^{2-}} = 0,1$ nên $SO_4^{2-}$ dư
$n_{BaSO_4} = n_{Ba^{2+}} = 0,05(mol)$
$m_{BaSO_4} = 0,05.233 = 11,65(gam)$
$n_{OH^-} = 0,1.0,5.2 + 0,1.0,5 = 0,15(mol)$
$n_{H^+} = 0,1.2 = 0,2(mol)$
$H^+ + OH^- \to H_2O$
$n_{H^+\ dư} = 0,2 - 0,15 = 0,05(mol)$
$V_{dd} = 0,1 + 0,1 + 0,1 = 0,3(lít)$
$[H^+] = \dfrac{0,05}{0,3} = \dfrac{1}{6}M$
$pH = -log( \dfrac{1}{6} ) = 0,778$
\(n_{Ba^{2+}}=0.1\cdot0.5=0.05\left(mol\right)\)
\(n_{OH^-}=0.1\cdot0.5\cdot2+0.1\cdot0.5=0.15\left(mol\right)\)
\(n_{H^+}=2\cdot0.1\cdot1=0.2\left(mol\right)\)
\(n_{SO_4^{2-}}=0.1\left(mol\right)\)
\(Ba^{2+}+SO_4^{2-}\rightarrow BaSO_4\)
\(0.05.........0.05.............0.05\)
\(SO_4^{2-}dư\)
\(m_{\downarrow}=0.05\cdot233=11.65\left(g\right)\)
\(H^++OH^-\rightarrow H_2O\)
\(0.15.......0.15\)
\(n_{H^+\left(dư\right)}=0.2-0.15=0.05\left(mol\right)\)
\(\left[H^+\right]=\dfrac{0.05}{0.1+0.1+0.1}=\dfrac{1}{6}\)
\(pH=-log\left(\dfrac{1}{6}\right)=0.77\)

a, \(NaHCO_3+NaOH\rightarrow Na_2CO_3+H_2O\)
\(HCO_3^-+OH^-\rightarrow CO_3^{2-}+H_2O\)
b, \(\left[Na^+\right]=\dfrac{0,2.1,5+0,12.1,6}{0,2+0,12}=1,5376M\)
\(\left[CO_3^{2-}\right]=\dfrac{0,2.1,5}{0,2+0,12}=0,9375M\)
\(n_{H^+}=0,3\left(mol\right)\)
\(n_{OH^-}=0,192\left(mol\right)\)
\(\Rightarrow n_{H^+dư}=0,108\left(mol\right)\)
\(\Rightarrow\left[H^+\right]=\dfrac{0,108}{0,2+0,12}=0,3375M\)
\(\Rightarrow pH\approx0,47\)

\(n_{NaOH}=0.2\cdot0.5=0.1\left(mol\right)\)
\(n_{CuSO_4}=0.1\cdot2=0.2\left(mol\right)\)
\(2NaOH+CuSO_4\rightarrow Na_2SO_4+Cu\left(OH\right)_2\)
\(0.1.............0.05...............0.05...........0.05\)
\(m_{Cu\left(OH\right)_2}=0.05\cdot98=4.9\left(g\right)\)
\(C_{M_{Na_2SO_4}}=\dfrac{0.05}{0.2+0.1}=0.167\left(M\right)\)
\(C_{M_{CuSO_4\left(dư\right)}}=\dfrac{0.2-0.05}{0.1}=1.5\left(M\right)\)

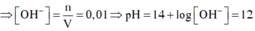
a)
$NaOH + HCl \to NaCl + H_2O$
$OH^- + H^+ \to H_2O$
b)
$n_{HCl} = 0,1.0,01 = 0,001(mol)$
$n_{NaOH} = 0,2.0,5= 0,1(mol)$
$\Rightarrow$ NaOH dư, HCl hết
$n_{NaOH\ pư} = 0,001 \Rightarrow n_{NaOH\ dư} = 0,1 - 0,001 = 0,099(mol)$
$\Rightarrow [OH^-] = \dfrac{0,099}{0,1 + 0,2} = 0,33M$
$\Rightarrow pOH = -log(0,33) = 0,48 $
$pH = 14 - pOH = 14 - 0,48 = 13,52$