2x - 1 = 1023
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


\(A=x^2+x+2=\left(x^2+x+\frac{1}{4}\right)+\frac{7}{4}=\left(x+\frac{1}{2}\right)^2+\frac{7}{4}\ge0+\frac{7}{4}=\frac{7}{4}.\) Dâu bàng xay ra khi: \(x=\frac{-1}{2}\)
\(B=4x^2-4x-1=\left(4x^2-4x+1\right)-2=\left(2x-1\right)^2-2\ge0-2=-2\Rightarrow B_{min}=-2\) Dâu bàng xay ra: \(x=\frac{1}{2}\)
\(C=x^2+y^2+2x-4y+2=x^2+y^2+2x-4y+5-3=\left(x^2+2x+1\right)+\left(y^2-4y+4\right)-3=\left(x+1\right)^2+\left(y-2\right)^2-3\ge0+0-3=-3\) Dâu bàng xay ra\(\Leftrightarrow\left\{{}\begin{matrix}x+1=0\\y-2=0\end{matrix}\right.\Leftrightarrow}\left\{{}\begin{matrix}x=-1\\y=2\end{matrix}\right.\)

Khối lượng bằng gam của:
- 6,02. 10 23 phân tử nước: 6,02. 10 23 .18.1,66. 10 - 24 = 17,988(g) ≈ 18(g)
- 6,02. 10 23 phân tử C O 2 : 6,02. 10 23 .44.1,66. 10 - 24 = 43,97(g) ≈ 44(g).
- 6,02. 10 23 phân tử C a C O 3 : 6,02. 10 23 .100. 1,66. 10 - 24 = 99,9(g) ≈ 100(g).

24.
10
23
phân tử
H
2
O
=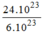 = 4(mol) phân tử
H
2
O
= 4(mol) phân tử
H
2
O
1,44.
10
23
phân tử
C
O
2
=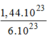 = 0,24(mol) phân tử
C
O
2
.
= 0,24(mol) phân tử
C
O
2
.
0,66.
10
23
phân tử
C
12
H
22
O
11
=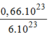 = 0,11(mol) phân tử
C
12
H
22
O
11
.
= 0,11(mol) phân tử
C
12
H
22
O
11
.

\(1.m_{Cu}=1,2.64=76,8\left(g\right)\\ 2.m_{NaCl}=1,25.58,5=73,125\\ 3.n_{C_6H_{12}O_6}=\dfrac{7,2.10^{23}}{6.10^{23}}=1,2\left(mol\right)\\ \Rightarrow m_{C_6H_{12}O_6}=1,2.180=216\left(g\right)\\ 4.n_{O_2}=3,6.32=115,2\left(g\right)\\ 5.n_{O_2}=\dfrac{1,2.10^{23}}{6.10^{23}}=0,2\left(mol\right)\\ \Rightarrow m_{O_2}=0,2.32=6,4\left(g\right)\\ 6.n_{N_2}=\dfrac{26,88}{22,4}=1,2\left(mol\right)\\ \Rightarrow m_{N_2}=1,2.28=33,6\left(g\right)\\ 7.n_{CO_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\\ \Rightarrow m_{CO_2}=0,5.44=22\left(g\right)\\ 8.n_{H_2}=\dfrac{31,36}{22,4}=1,4\left(mol\right)\\ \Rightarrow m_{H_2}=1,4.2=2,8\left(g\right)\)
\(1,m_{Cu}=1,2\cdot64=76,8\left(g\right)\\ 2,m_{NaCl}=1,25\cdot58,5=73,125\left(g\right)\\ 3,n_{C_6H_{12}O_6}=\dfrac{7,2\cdot10^{-23}}{6\cdot10^{-23}}=1,2\left(mol\right)\\ \Rightarrow m_{C_6H_{12}O_6}=1,2\cdot180=216\left(g\right)\\ 4,m_{O_2}=3,6\cdot32=115,2\left(g\right)\\ 5,n_{O_2}=\dfrac{1,2\cdot10^{-23}}{6\cdot10^{-23}}=0,2\left(mol\right)\\ \Rightarrow m_{O_2}=0,2\cdot32=6,4\left(g\right)\\ 6,n_{N_2}=\dfrac{26,88}{22,4}=1,2\left(mol\right)\\ \Rightarrow m_{N_2}=1,2\cdot28=33,6\left(g\right)\\ 7,n_{CO_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\\ \Rightarrow m_{CO_2}=0,5\cdot44=22\left(g\right)\\ 8,n_{H_2}=\dfrac{31,36}{22,4}=1,4\left(mol\right)\\ \Rightarrow m_{H_2}=1,4\cdot2=2,8\left(g\right)\)

\(C=\frac{1}{3}+\frac{1}{15}+\frac{1}{35}+...+\frac{1}{1023}\)
\(=\frac{1}{1\cdot3}+\frac{1}{3\cdot5}+\frac{1}{5\cdot7}....+\frac{1}{31\cdot33}\)
\(=\frac{1}{2}\left(1-\frac{1}{3}+\frac{1}{3}-\frac{1}{5}+...+\frac{1}{31}-\frac{1}{33}\right)\)
\(=\frac{1}{2}\left(1-\frac{1}{33}\right)\)
\(=\frac{1}{2}\cdot\frac{32}{33}\)
\(=\frac{32}{66}=\frac{16}{33}\)
Vậy \(A=\frac{16}{33}\)
HOK TỐT .
\(C=\frac{1}{3}+\frac{1}{15}+\frac{1}{35}+...+\frac{1}{1023}\)
\(C=\frac{1}{1\cdot3}+\frac{1}{3\cdot5}+\frac{1}{5\cdot7}+...+\frac{1}{31\cdot33}\)
\(C=\frac{1}{2}\left(\frac{2}{1\cdot3}+\frac{2}{3\cdot5}+\frac{2}{5\cdot7}+...+\frac{2}{31\cdot33}\right)\)
\(C=\frac{1}{2}\left(1-\frac{1}{3}+\frac{1}{3}-\frac{1}{5}+\frac{1}{5}-\frac{1}{7}+...+\frac{1}{31}-\frac{1}{33}\right)\)
\(C=\frac{1}{2}\left(1-\frac{1}{33}\right)\)
\(C=\frac{1}{2}\cdot\frac{32}{33}\)
\(C=\frac{16}{33}\)
2x - 1 = 1023
=> 2x = 1024
=> 2x = \(2^{10}\)
=> x = 10