Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

nZn=0,1 mol
Zn +2HCl=> ZnCl2+ H2
0,1 mol =>0,2 mol
=>mHCl=36,5.0,2=7,3g
=>m dd HCl=7,3/14,6%=50g
mdd sau pứ=6,5+50-0,1.2=56,3g
=>C% dd ZnCl2=(0,1.136)/56,3.100%=24,16%
a.b. Zn + 2HCl ---> ZnCl2 + H2 (1)
Theo pt: 65g 73g 136g 2g
Theo đề: 6,5g 7,3g 13,6g
=> mddHCl=\(\frac{7,3.100}{14,6}=50\left(g\right)\)
c. Từ pt (1), ta có: \(C_{\%}=\frac{13,6}{50+6,5}.100\%=24,1\%\)
![]()

a. \(n_{Zn}=\dfrac{6.5}{65}=0,1\left(mol\right)\)
PTHH : Zn + 2HCl -> ZnCl2 + H2
0,1 0,2 0,1
b. \(V_{H_2}=0,1.22,4=2,24\left(l\right)\)
c. \(m_{HCl}=0,2.36,5=7,3\left(g\right)\)
\(n_{Zn}=\dfrac{6,5}{65}=0,1mol\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
0,1 0,2 0,1
\(V_{H_2}=0,1\cdot22,4=2,24l\)
\(m_{HCl}=0,2\cdot36,5=7,3g\)

\(n_{Zn}=\dfrac{6.5}{65}=0.1\left(mol\right)\)
\(n_{H_2SO_4}=0.3\cdot1=0.3\left(mol\right)\)
\(Zn+H_2SO_4\rightarrow ZnSO_4+H_2\)
\(0.1........0.1...........0.1.......0.1\)
\(\Rightarrow H_2SO_4dư\)
\(n_{H_2SO_4\left(dư\right)}=0.3-0.1=0.2\left(mol\right)\)
\(n_{ZnSO_4}=n_{H_2}=0.1\left(mol\right)\)
\(C_{M_{ZnSO_4}}=\dfrac{0.1}{0.3}=0.33\left(M\right)\)
\(C_{M_{H_2SO_4\left(dư\right)}}=\dfrac{0.2}{0.3}=0.66\left(M\right)\)
\(a) Zn + H_2SO_4 \to ZnSO_4 + H_2\\ b) n_{Zn} = \dfrac{6,5}{65} = 0,1 < n_{H_2SO_4} =0,3 \to H_2SO_4\ dư\\ n_{H_2SO_4\ pư} = n_{ZnSO_4} = n_{Zn} = 0,1(mol)\\ n_{H_2SO_4\ dư} = 0,3 - 0,1 = 0,2(mol)\\ c) C_{M_{ZnSO_4}} = \dfrac{0,1}{0,3} = 0,33M\\ C_{M_{H_2SO_4}} = \dfrac{0,2}{0,3} = 0,67M\)

\(a.Fe+2HCl\rightarrow FeCl_2+H_2\\ b.n_{Fe}=\dfrac{5,6}{56}=0,1\left(mol\right)\\ TheoPT:n_{HCl}=2n_{Fe}=0,2\left(mol\right)\\ \Rightarrow CM_{HCl}=\dfrac{0,2}{0,2}=1M\)

a, \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
b, \(n_{Zn}=\dfrac{13}{65}=0,2\left(mol\right)\)
Theo PT: \(n_{H_2}=n_{Zn}=0,2\left(mol\right)\Rightarrow V_{H_2}=0,2.22,4=4,48\left(l\right)\)
c, \(n_{ZnCl_2}=n_{Zn}=0,2\left(mol\right)\Rightarrow m_{ZnCl_2}=0,2.136=27,2\left(g\right)\)
d, \(n_{HCl}=2n_{Zn}=0,4\left(mol\right)\Rightarrow m_{HCl}=0,4.36,5=14,6\left(g\right)\)
\(\Rightarrow m_{ddHCl}=\dfrac{14,6}{7,3\%}=200\left(g\right)\)
⇒ m dd sau pư = 13 + 200 - 0,2.2 = 212,6 (g)
\(\Rightarrow C\%_{ZnCl_2}=\dfrac{27,2}{212,6}.100\%\approx12,79\%\)

a, \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
b, \(n_{Zn}=\dfrac{13}{65}=0,2\left(mol\right)\)
Theo PT: \(n_{H_2}=n_{Zn}=0,2\left(mol\right)\Rightarrow V_{H_2}=0,2.22,4=4,48\left(l\right)\)
c, \(n_{HCl}=2n_{Zn}=0,4\left(mol\right)\Rightarrow m_{HCl}=0,4.36,5=14,6\left(g\right)\)
\(\Rightarrow m_{ddHCl}=\dfrac{14,6}{10,95\%}=\dfrac{400}{3}\left(g\right)\)
d, \(n_{ZnCl_2}=n_{Zn}=0,2\left(mol\right)\)
Ta có: m dd sau pư = 13 + 400/3 - 0,2.2 = 2189/15 (g)
\(\Rightarrow C\%_{ZnCl_2}=\dfrac{0,2.136}{\dfrac{2189}{15}}.100\%\approx18,64\%\)
a.
PTHH:
Zn + 2HCl ---> ZnCl2 + H2
0.2 0.4 0.2 0.2 (mol)
b.
nZn=13/65=0.2(mol)
V H2 = 0.2*22.4 = 4.48 (l)
c.
mHCl=0.4*36.5=14.6(g)
mddHCl=14.6/10.95*100~133(g)
d.
mZn=0.2*35.5=7.1(g)
mZnCl2=0.2*106=21.2(g)
mH2=0.2*2=0.4(g)
Theo ĐLBTKL, ta có:
mZn + mddHCl = mddZnCl2 + mH2
7.1 + 133 = mddZnCl2 + 4
=> mddZnCl2= 7.1 + 133 - 4 = 136.1 (g)
S ZnCl2= 21.2/136.1*100 ~ 15 (g)

a)nMgO=0,15(mol)
Ta có PTHH:
MgO+H2SO4->MgSO4+H2O
0,15......0,15...........0,15..................(mol)
Theo PTHH:mH2SO4=0,15.98=14,7g
b)Ta có:mddH2SO4=1,2.50=60(g)
=>Nồng độ % dd H2SO4là:
C%ddH2SO4=\(\dfrac{14,7}{60}100\)=24,5%
c)Theo PTHH:mMgSO4=0,15.120=18(g)
Khối lượng dd sau pư là:
mddsau=6+60=66(g)
Vậy nồng độ % dd sau pư là:
C%ddsau=\(\dfrac{18}{66}.100\)=27,27%
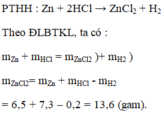
\(n_{Zn}=\dfrac{6,5}{65}=0,1\left(mol\right)\)
a) Pt : \(Zn+2HCl\rightarrow ZnCl_2+H_2|\)
1 2 1 1
0,1 0,2 0,1
b) \(n_{HCl}=\dfrac{0,1.2}{1}=0,2\left(mol\right)\)
\(V_{ddHCl}=\dfrac{0,2}{1}=0,2\left(l\right)\)
c) \(n_{ZnCl2}=\dfrac{0,2.1}{2}=0,1\left(mol\right)\)
\(C_{M_{ZnCl2}}=\dfrac{0,1}{0,2}=0,5\left(M\right)\)
Chúc bạn học tốt
\(a/\\ Zn+2HCl \to ZnCl_2+H_2\\ b/\\ n_{Zn}=0,1(mol)\\ n_{HCl}=0,2(mol)\\ V_{HCl}=\frac{0,2}{1}=0,2(l)\\ c/\\ n_{ZnCl_2}=0,1(mol)\\ CM_{ZnCl_2}=\frac{0,1}{0,2}=0,5M\)