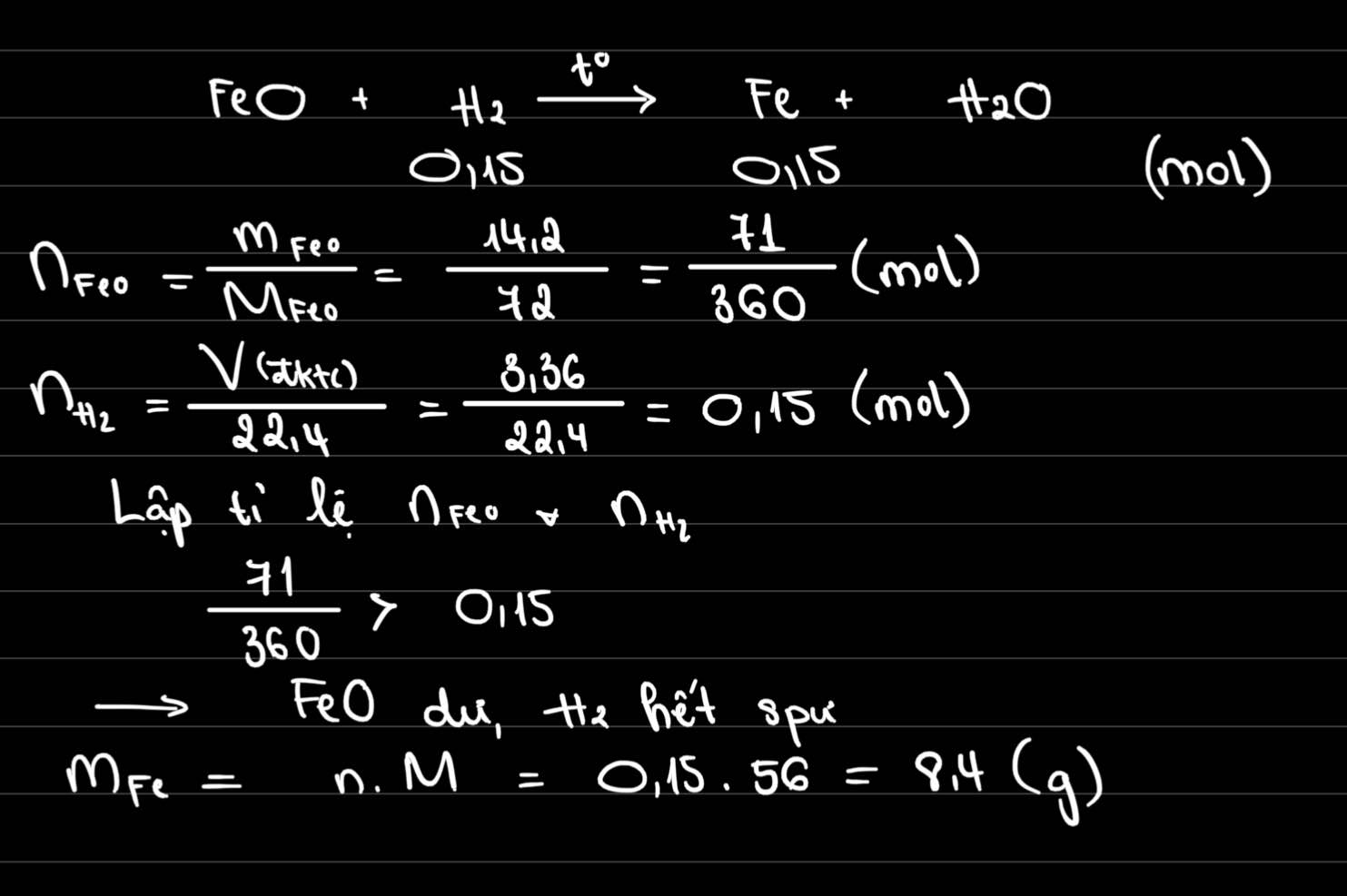Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


nH2 = \(\dfrac{3,6}{22,4}\) = 0,16 mol
nFe2O3 = \(\dfrac{32}{160}\) = 0,2 mol
3H2 + Fe2O3 -> 2Fe + 3H2O
0,16(hết);0,2(dư) ->0,106 ->0,16
mH2O = 0,106 . 18 = 1,908 g
mFe = 0,16.56 = 8,96 g
a)3H2 + Fe2O3 -> 2Fe + 3H2O
b)nH2 = 3,6/22,4 \(\simeq\)0,16 (mol)
nFe2O3 = 32/160 = 0,2 (mol)
ta có: 0,16/3 < 0,2/1 => H2 hết, Fe2O3 dư
theo Pt ta có: nFe = 2/3.nH2=2/3.0,16\(\simeq\)0,1( mol)
n H2O=nH2=0,16 (mol)
=> m Fe=0,1.56=5,6(g)
mH2O=0,16.18=2,88(g)

\(a.n_{Fe_2O_3}=\dfrac{24}{160}=0,15\left(mol\right)\\ n_{H_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\\ Fe_2O_3+3H_2\underrightarrow{to}2Fe+3H_2O\\ Vì:\dfrac{0,3}{3}< \dfrac{0,15}{1}\\ \rightarrow Fe_2O_3dư\\ n_{Fe_2O_3\left(dư\right)}=0,15-\dfrac{0,3}{3}=0,05\left(mol\right)\\ m_{Fe_2O_3\left(dư\right)}=0,05.160=8\left(g\right)\\ b.n_{Fe}=\dfrac{0,3}{3}.2=0,2\left(mol\right)\\ m_{Fe}=0,2.56=11,2\left(g\right)\\ c.m_{rắn}=m_{Fe}+m_{Fe_2O_3\left(dư\right)}=11,2+8=19,2\left(g\right)\)

nH2 = 3.36/22.4 = 0.15 (mol)
nFe2O3 = 40/160 = 0.25 (mol)
Fe2O3 + 3H2 -t0-> 2Fe + 3H2O
Bđ: 0.25.......0.15
Pư: 0.05.......0.15.........0.1.......0.15
Kt: 0.2.............0............0.1.......0.15
mCr = mFe2O3(dư) + mFe = 0.2*160 + 0.1*56 = 37.6 (g)
mH2O = 0.15 * 18 = 2.7 (g)

\(a)Fe_3O_4 + 4H_2 \xrightarrow{t^o} 3Fe + 4H_2O\\ b)n_{H_2} = \dfrac{8,96}{22,4} = 0,4(mol)\\ n_{Fe} = \dfrac{3}{4}n_{H_2} = 0,3(mol)\\ n_{Fe_3O_4\ pư} = \dfrac{1}{4}n_{H_2} = 0,1(mol)\\ \Rightarrow m_{chất\ rắn\ sau\ phản\ ứng} = 0,3.56 + (34,8 -0,1.232)=28,4(gam)\\ c) \%m_{Fe_3O_4\ bị\ khử} = \dfrac{0,1.232}{34,8}.100\% = 66,67\%\)

\(n_{CuO}=2a\left(mol\right)\Rightarrow n_{Fe_2O_3}=a\left(mol\right)\)
\(m_X=80\cdot2a+160a=80\left(g\right)\)
\(\Rightarrow a=0.25\left(mol\right)\)
\(CuO+H_2\underrightarrow{^{^{t^0}}}Cu+H_2O\)
\(Fe_2O_3+3H_2\underrightarrow{^{^{t^0}}}2Fe+3H_2O\)
\(n_{H_2}=0.5+0.25\cdot3=1.25\left(mol\right)\)
\(V_{H_2}=1.25\cdot22.4=28\left(l\right)\)
\(m_{cr}=0.5\cdot64+0.5\cdot56=60\left(g\right)\)

\(a.n_{CuO}=\dfrac{40}{80}=0,5\left(mol\right)\\ n_{H_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\\ PTHH:CuO+H_2\underrightarrow{t^o}Cu+H_2O\\ Vì:\dfrac{0,15}{1}< \dfrac{0,5}{1}\\ \rightarrow CuOdư\\ n_{CuO\left(p.ứ\right)}=n_{Cu}=n_{H_2}=0,15\left(mol\right)\\ \rightarrow n_{CuO\left(dư\right)}=0,5-0,15=0,35\left(mol\right)\\ m_{CuO\left(DƯ\right)}=0,35.80=28\left(g\right)\\ b.m_{Cu}=0,35.64=22,4\left(g\right)\\ c.m_{hh_{rắn}}=m_{Cu}+m_{CuO\left(dư\right)}=22,4+28=50,4\left(g\right)\)

\( CuO+H_2\rightarrow\left(t^o\right)Cu+H_2O\\ n_{H_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\\ n_{CuO}=\dfrac{48}{80}=0,6\left(mol\right)\\ Vì:\dfrac{0,5}{1}< \dfrac{0,6}{1}\Rightarrow CuO\left(dư\right)\Rightarrow Tính.theo.n_{H_2}\\ Đặt:a=n_{CuO\left(p.ứ\right)}\\ m_{rắn}=41,6\left(g\right)\\ \Leftrightarrow64a+80.\left(0,6-a\right)=41,6\\ \Leftrightarrow a=0,4\left(mol\right)\\ n_{CuO\left(LT\right)}=n_{H_2}=0,5\left(mol\right)\\ \Rightarrow H=\dfrac{n_{CuO\left(TT\right)}}{n_{CuO\left(LT\right)}}.100\%=\dfrac{0,4}{0,5}.100=80\%\)
Thể tích H2 phản ứng: 11,2 (lít) (đề bài)
\( \%m_{CuO\left(p.ứ\right)}=\dfrac{0,4}{0,6}.100\%=66,667\%\) (Do số mol tỉ lệ thuận với khối lượng)

`FeO + H_2` $\xrightarrow[]{t^o}$ `Fe + H_2 O`
`a) n_[H_2] = [ 3,36 ] / [ 22,4 ] = 0,15 (mol)`
`n_[FeO] = [ 14,2 ] / 72 = 71 / 360`
Ta có: `[ 0,15 ] / 1 < [ 71 / 360 ] / 1`
`=> FeO` dư
Theo `PTHH` có: `n_[FeO_\text{(p/ứ)}] = n_[H_2] = 0,15 (mol)`
`=> n_[FeO_\text{(dư)}] = 71 / 360 - 0,15 = 17 / 360 (mol)`
_______________________________________________
`b)` Theo `PTHH` có: `n_[Fe] = n_[H_2] = 0,15 (mol)`
`=> m_[Fe] = 0,15 . 56 = 8,4 (g)`