G giúp mink vs
giúp mink vs
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

BT: Đốt cháy hoàn toàn 12(g) C trong oxi dư
a) mo2 phản ứng = ?
b) Vco2 tạo ra = ?
giúp mink vs mn ơi !

\(n_C=\dfrac{m}{M}=\dfrac{12}{12}=1\left(mol\right)\\ PTHH:C+O_2-^{t^o}>CO_2\)
tỉ lệ 1 : 1 : 1
n(mol) 1---->1----------->1
\(m_{O_2}=n\cdot M=1\cdot32=32\left(g\right)\\ V_{CO_2\left(dktc\right)}=n\cdot22,4=1\cdot22,4=22,4\left(l\right)\)
Ta có: \(n_C=\dfrac{12}{12}=1\left(mol\right)\)
PT: \(C+O_2\underrightarrow{t^o}CO_2\)
____1____1____1 (mol)
a, \(m_{O_2}=1.32=32\left(g\right)\)
b, \(V_{CO_2}=1.22,4=22,4\left(l\right)\)

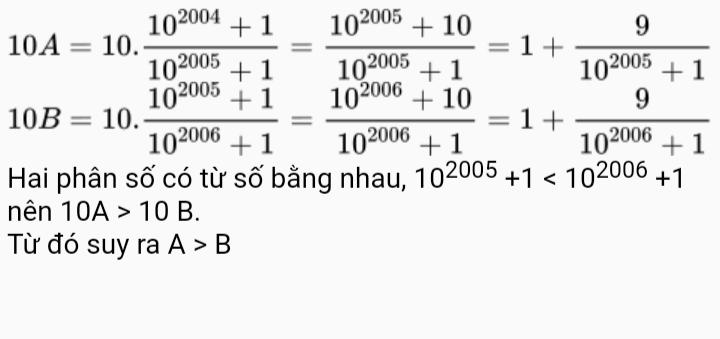
a) \(\dfrac{35}{101}=\dfrac{105}{303}< \dfrac{189}{303}\Rightarrow\dfrac{35}{101}< \dfrac{189}{303}\)
b) \(\dfrac{11}{13}< \dfrac{11+2}{13+2}=\dfrac{13}{15}< \dfrac{14}{15}\Rightarrow\dfrac{11}{-13}>\dfrac{-14}{15}\)
c) \(-\dfrac{32}{19}< 0< \dfrac{23}{32}\Rightarrow-\dfrac{32}{19}< \dfrac{23}{32}\)
d) \(1,561< 1,5661\Rightarrow-1,561>-1,5661\)
e) \(0,1=\dfrac{1}{10}=\dfrac{40}{400}< \dfrac{40+56}{400+56}=\dfrac{96}{456}< \dfrac{176}{456}\Rightarrow0,1< \dfrac{176}{456}\)
g) \(0,3=\dfrac{3}{10}=\dfrac{9}{30}< \dfrac{9+8}{30+8}=\dfrac{17}{38}< \dfrac{19}{38}\Rightarrow0,3< \dfrac{19}{38}\Rightarrow-0,3>\dfrac{-19}{38}\)




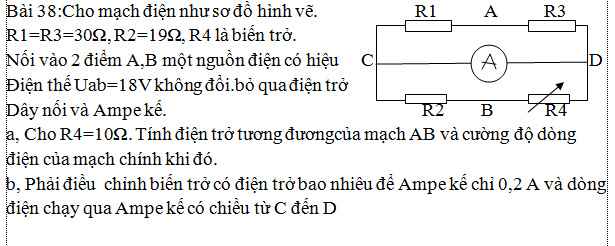
TH1: \(R1ntR2=>Rtd=R1+R2=90\left(om\right)\left(1\right)\)
TH2: \(R1//R2=>Rtd=\dfrac{R1.R2}{R1+R2}=20\left(om\right)\left(2\right)\)
(1)(2)=>hệ pt: \(\left\{{}\begin{matrix}R1+R2=90\\\dfrac{R1.R2}{R1+R2}=20\end{matrix}\right.=>\left[{}\begin{matrix}\left\{{}\begin{matrix}R1=30\left(om\right)\\R2=60\left(om\right)\end{matrix}\right.\\\left\{{}\begin{matrix}R1=60\left(om\right)\\R2=30\left(om\right)\end{matrix}\right.\end{matrix}\right.\)
vậy ....................
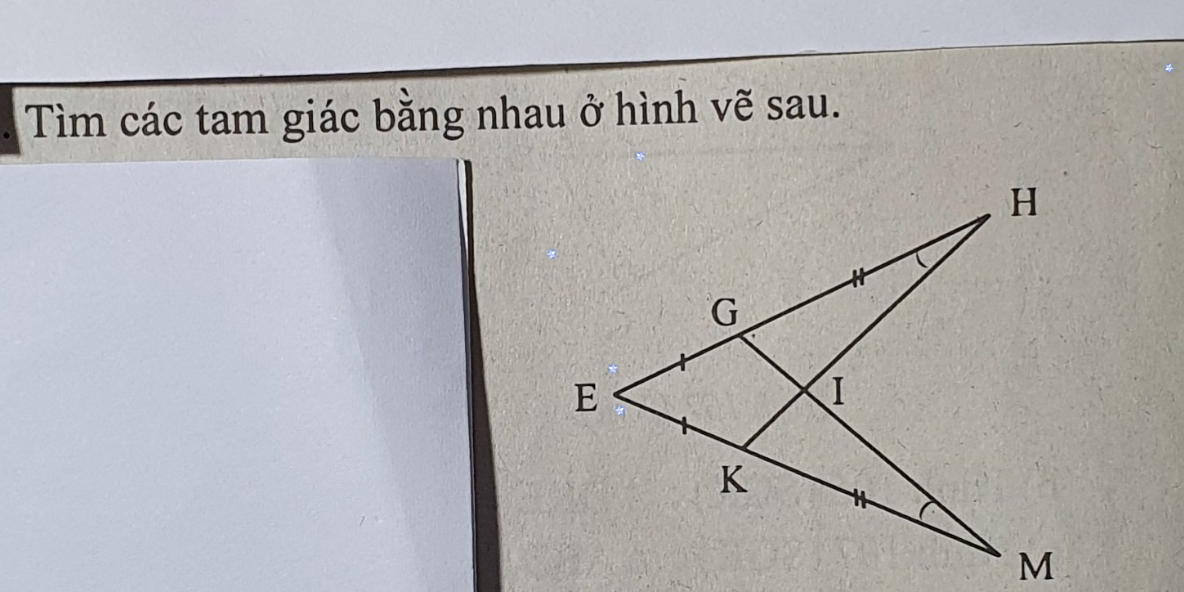
 giúp mink vs
giúp mink vs
 giúp mink giải vs
giúp mink giải vs
V
1 A
2 D
3 B
4 A
5 C
Bài 2
1 C
2 D
3 C
4 A
5 A