 Giúp mình câu 30
Giúp mình câu 30
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


21. didn’t go
22. isn’t painting
23. aren’t planting
24. Are they making
25. is repairing
26. is taking
27. is explaining
28. is studying
29. tries/ don’t think
30. passes

30B 31B 32D 33D 34C 35B 36B 37C 38B 39A 40D 41D 42A 43D 44D 45A 46C 47B 48C 49B 50C 51C 52B 53D 54B 55C 56A 57C 58A 59D 60B




30.
\(V_{\left(O;\dfrac{3}{4}\right)}\left(M\right)=M_1\Rightarrow\left\{{}\begin{matrix}x_1=\dfrac{3}{4}.4=3\\y_1=\dfrac{3}{4}.1=\dfrac{3}{4}\end{matrix}\right.\)
\(Q_{\left(O;-90^0\right)}\left(M_1\right)=M'\Rightarrow\left\{{}\begin{matrix}x'=y_1=\dfrac{3}{4}\\y'=-x_1=-3\end{matrix}\right.\)
\(\Rightarrow M'\left(\dfrac{3}{4};-3\right)\)
31.
\(cos\dfrac{x}{2}=-\dfrac{\sqrt{3}}{2}\Rightarrow\dfrac{x}{2}=\pm\dfrac{5\pi}{6}+k2\pi\)
\(\Rightarrow x=\pm\dfrac{5\pi}{3}+k4\pi\)
Nghiệm dương nhỏ nhất \(x=\dfrac{5\pi}{3}\Rightarrow a+b=5+3=8\)

\(\dfrac{x-7}{\left(x+2\right)\left(x-3\right)}>1\Leftrightarrow\dfrac{x-7-\left(x+2\right)\left(x-3\right)}{\left(x+2\right)\left(x-3\right)}>0\)
\(\Leftrightarrow\dfrac{-\left(x-1\right)^2}{\left(x+2\right)\left(x-3\right)}>0\Rightarrow x\in\left(-2;3\right)\backslash\left\{1\right\}\)
\(\Rightarrow2c+a-3b=2.1+\left(-2\right)-3.3=-9\)
30
\(=sin\left(\dfrac{5\pi}{12}\right).sin\left(2x+\dfrac{\pi}{12}\right)-sin\dfrac{\pi}{12}.cos\left(2x+\dfrac{\pi}{12}\right)\)
\(=cos\left(\dfrac{\pi}{2}-\dfrac{5\pi}{12}\right)sin\left(2x+\dfrac{\pi}{12}\right)-sin\left(\dfrac{\pi}{12}\right)cos\left(2x+\dfrac{\pi}{12}\right)\)
\(=sin\left(2x+\dfrac{\pi}{12}\right)cos\left(\dfrac{\pi}{12}\right)-cos\left(2x+\dfrac{\pi}{12}\right)sin\left(\dfrac{\pi}{12}\right)\)
\(=sin\left(2x+\dfrac{\pi}{12}-\dfrac{\pi}{12}\right)=sin2x\)
\(\Rightarrow\left\{{}\begin{matrix}a=2\\b=0\end{matrix}\right.\) \(\Rightarrow b-2a=-4\)







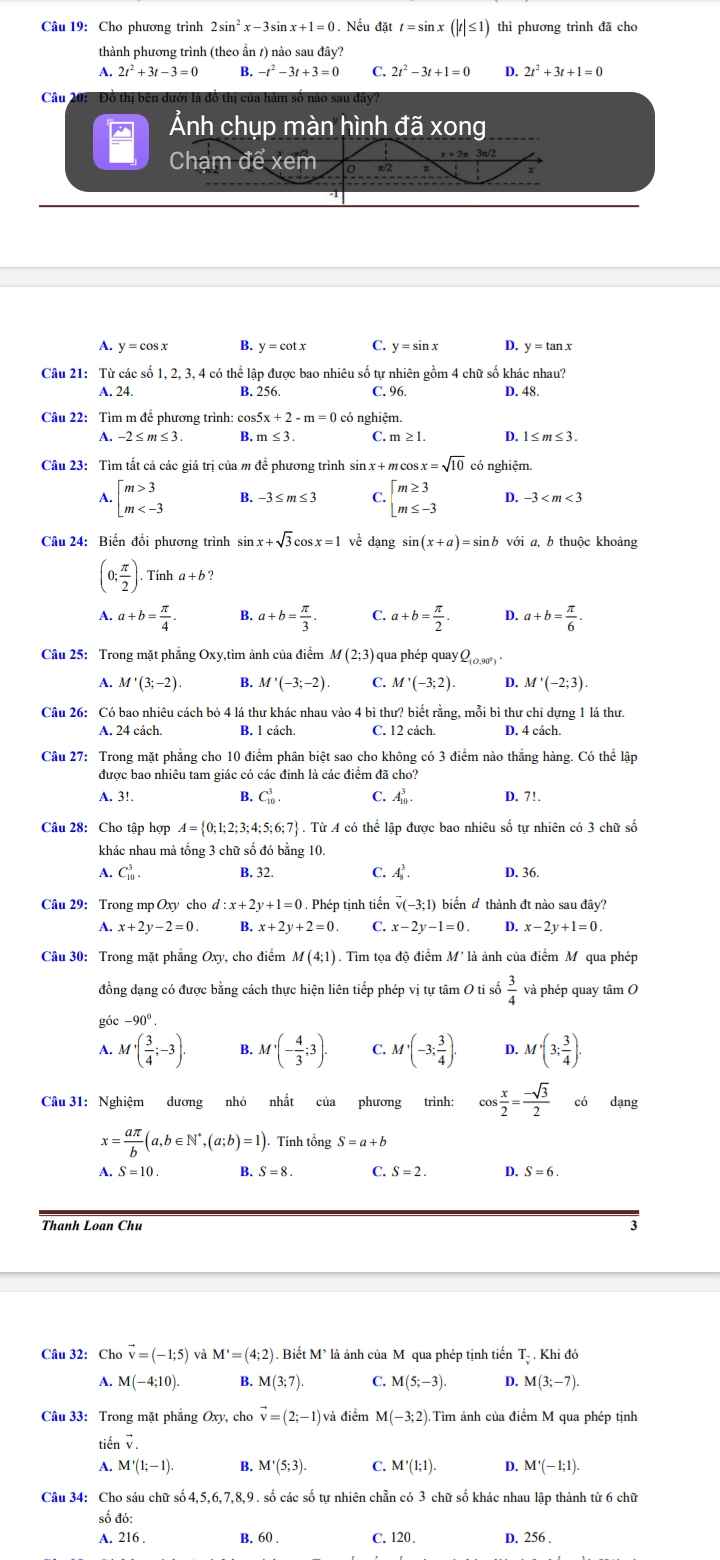

$(1) CH_3COOH + CH_3OH \buildrel{{H_2SO_{4\ đ},t^o}}\over\rightleftharpoons CH_3COOCH_3 + H_2O$
$(2) CH_3OH + CuO \xrightarrow{t^o} HCHO + Cu + H_2O$
$(3)$ Không phản ứng
$(4) C_6H_5OH + Na \to C_6H_5ONa + \dfrac{1}{2}H_2$
$(5) C_6H_5OH + 3HNO_{3\ đ} \to C_6H_2(NO_3)_3OH + 3H_2O$
$(6) CH_3OH + HCOOH \buildrel{{H_2SO_{4\ đ},t^o}}\over\rightleftharpoons HCOOCH_3 + H_2O$
$(7) CH_3CH_2OH + CuO \xrightarrow{t^o} CH_3CHO + Cu + H_2O$
$(8) Không phản ứng
$(9) C_6H_5OH + KOH \to C_6H_5OK + H_2O$
$(10) C_6H_5OH + 3Br_2 \to C_6H_2Br_3OH + 3HBr$