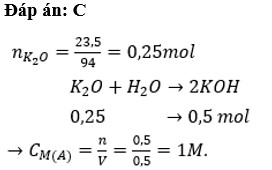hoà tan 2,24 lít NH3 vào 100 mm lít nước . Tính nồng độ mol dung dịch sau hoà tan ( biết các lí do ở điệu kiên tiêu chuẩn)
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


\(n_{Na_2O}=\dfrac{15,5}{62}=0,25\left(mol\right)\\ PTHH:Na_2O+H_2O\rightarrow2NaOH\\ n_{NaOH}=2.0,25=0,5\left(mol\right)\\ a,C_{MddNaOH}=\dfrac{0,5}{0,5}=1\left(M\right)\\ b,2NaOH+H_2SO_4\rightarrow Na_2SO_4+2H_2O\\ n_{H_2SO_4}=n_{Na_2SO_4}=\dfrac{0,5}{2}=0,25\left(mol\right)\\ m_{H_2SO_4}=0,25.98=24,5\left(g\right)\\ m_{ddH_2SO_4}=\dfrac{24,5.100}{20}=122,5\left(g\right)\\ V_{ddH_2SO_4}=\dfrac{122,5}{1,14}\approx107,456\left(ml\right)\\ c,V_{ddsau}=V_{ddNaOH}+V_{ddH_2SO_4}\approx0,5+0,107456=0,607456\left(l\right)\\C_{MddNa_2SO_4}\approx\dfrac{ 0,25}{0,607456}\approx0,411552\left(M\right)\)

1)
$n_{Na_2O} = \dfrac{6,2}{62} = 0,1(mol)$
$Na_2O + H_2O \to 2NaOH$
$n_{NaOH} = 2n_{Na_2O} = 0,2(mol)$
$m_{dd} = 6,2 + 193,8 = 200(gam) \Rightarrow C\%_{NaOH} = \dfrac{0,2.40}{200}.100\% = 4\%$
2)
$n_{K_2O} = \dfrac{23,5}{94} = 0,25(mol)$
$K_2O + H_2O \to 2KOH$
$n_{KOH} = 2n_{K_2O} = 0,5(mol) \Rightarrow C_{M_{KOH}} = \dfrac{0,5}{0,5} = 1M$
3) $n_{Na_2O} = \dfrac{12,4}{62} = 0,2(mol)$
$Na_2O + H_2O \to 2NaOH$
$n_{NaOH} = 2n_{Na_2O} = 0,4(mol)$
$C_{M_{NaOH}} = \dfrac{0,4}{0,5} =0,8M$
4)
$Na_2SO_3 + 2HCl \to 2NaCl +S O_2 + H_2O$
Theo PTHH :
$n_{SO_2} = n_{Na_2SO_3} = \dfrac{12,6}{126} = 0,1(mol)$
$V_{SO_2} = 0,1.22,4 = 2,24(lít)$
5) $n_{CaO} = \dfrac{5,6}{56} = 0,1(mol)$
$CaO + 2HCl \to CaCl_2 + H_2O$
Theo PTHH :
$n_{HCl} = 2n_{CaO} = 0,2(mol) \Rightarrow m_{dd\ HCl} = \dfrac{0,2.36,5}{14,6\%} = 50(gam)$

a)
\(n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
b)
PTHH: 2R + 2H2O --> 2ROH + H2
_____0,2<--------------0,2<----0,1
=> \(M_R=\dfrac{7,8}{0,2}=39\left(K\right)\)
c)
\(C_{M\left(KOH\right)}=\dfrac{0,2}{0,5}=0,4M\)

\(1,C_{M\left(HCl\right)}=\dfrac{0,75}{0,5}=1,5M\\ 2,n_{Ca\left(OH\right)_2}=\dfrac{37}{74}=0,5\left(mol\right)\\ C_{M\left(Ca\left(OH\right)_2\right)}=\dfrac{0,5}{1,5}=0,33M\\ 3,n_{NaOH}=0,25+\dfrac{20}{40}=0,75\left(mol\right)\\ C_{M\left(NaOH\right)}=\dfrac{0,75}{2}=0,375M\\ 4,n_{H_2SO_4}=\dfrac{49}{98}=0,5\left(mol\right)\\ C_{M\left(H_2SO_4\right)}=\dfrac{0,5}{2}=0,25M\)

`1) C_[M_[HCl]] = [ 0,75 ] / [ 0,5 ] = 1,5 (M)`
_____________________________________________
`2)n_[Ca(OH)_2] = 37 / 74 = 0,5 (mol)`
`-> C_[M_[Ca(OH)_2]] = [ 0,5 ] / [ 1,5 ] ~~ 0,33 (M)`
_____________________________________________
`3) n_[NaOH] = 0,25 + 20 / 40 = 0,75 (mol)`
`-> C_[M_[NaOH]] = [ 0,75 ] / 2 = 0,375 (M)`
_____________________________________________
`4) n_[H_2 SO_4] = 49 / 98 = 0,5 (mol)`
`-> C_[M_[H_2 SO_4]] = [ 0,5 ] / 2 = 0,25 (M)`

a) nNa = 4,6/23 = 0,2 (mol)
PTHH: 2Na + 2H2O -> 2NaOH + H2
Mol: 0,2 ---> 0,2 ---> 0,2 ---> 0,1
VH2 = 0,1 . 22,4 = 2,24 (l)
b) CMNaOH = 0,2/0,1 = 2M
c) mH2O = 100 . 1 = 100 (g)
mNaOH = 0,2 . 40 = 8 (g)
mH2 = 0,1 . 2 = 0,2 (g)
mdd = 100 + 8 - 0,2 = 107,8 (g)
C%NaOH = 8/107,8 = 7,42%

\(n_{NaCl}=\dfrac{11,7}{58,5}=0,2\left(mol\right)\)
\(n_{NaNO_3}=\dfrac{100.8,5\%}{85}=0,1\left(mol\right)\)
\(V_{dd}=\dfrac{100}{1,25}=80\left(ml\right)\)
\(\left\{{}\begin{matrix}C_{M\left(NaCl\right)}=\dfrac{0,2}{0,08}=2,5M\\C_{M\left(NaNO_3\right)}=\dfrac{0,1}{0,08}=1,25M\end{matrix}\right.\)