Bài1:Hoà tan hoàn toàn x g Mg vừa đủ trong 110ml dd HCl 1,5M. Tính x? Bài2:Cho 20,25g Al tan hoàn toàn vừa đủ trong x g dd H2SO4 18,735 a,Tính x? b,Nồng độ % của dd thu đc sau phản ứng Bài3:Cho hỗn hợp X gồm 0,1mol Cu và 0,1mol K vào nước dư. Sau phản ứng thu đc dd A và m am chất rắn.Tính giá trị của m? Mong mọi người giải giúp em
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


\(a)2Al+6HCl\rightarrow2AlCl_3+3H_2\\ Fe+2HCl\rightarrow FeCl_2+H_2\\ b)n_{H_2}=\dfrac{5,6}{22,4}=0,25mol\\ n_{Al}=a;n_{Fe}=b\\ \left\{{}\begin{matrix}3a+b=0,25\\27a+56b=8,3\end{matrix}\right.\\ a=\dfrac{19}{470};b=\dfrac{121}{940}\\ \%m_{Al}=\dfrac{\dfrac{19}{470}\cdot27}{8,3}\cdot100=13,15\%\\ \%m_{Fe}=100-13,15=86,85\%\\ c)n_{HCl}=3\cdot\dfrac{19}{470}+2\cdot\dfrac{121}{940}=\dfrac{89}{235}mol\\ m_{ddHCl=}=\dfrac{\dfrac{89}{235}\cdot36,5}{7,3}\cdot100=189g\\ d)n_{AlCl_3}=n_{Al}=\dfrac{19}{470}mol\\ n_{Fe}=n_{FeCl_2}=\dfrac{121}{940}mol\)
\(m_{dd}=8,3+189-0,25.2=196,8g\\ C_{\%AlCl_3}=\dfrac{\dfrac{19}{470}\cdot133,8}{196,8}\cdot100=2,8\%\\ C_{\%FeCl_2}=\dfrac{\dfrac{121}{940}127}{196,8}\cdot100=8,3\%\)

a) Gọi số mol Al, Zn là a, b (mol)
=> 27a + 65b = 11,9 (1)
\(n_{H_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\)
PTHH: 2Al + 3H2SO4 --> Al2(SO4)3 + 3H2
a----->1,5a----------------->1,5a
Zn + H2SO4 --> ZnSO4 + H2
b------>b------------------>b
=> 1,5a + b = 0,4 (2)
(1)(2) => a = 0,2 (mol); b = 0,1 (mol)
\(\left\{{}\begin{matrix}\%m_{Al}=\dfrac{0,2.27}{11,9}.100\%=45,378\%\\\%m_{Zn}=\dfrac{0,1.65}{11,9}.100\%=54,622\%\end{matrix}\right.\)
b) nH2SO4 = 1,5a + b = 0,4 (mol)
=> mH2SO4 = 0,4.98 = 39,2 (g)
=> \(C\%_{dd.H_2SO_4}=\dfrac{39,2}{150}.100\%=26,133\%\)

\(n_{Mg}=\dfrac{4,8}{24}=0,2\left(mol\right)\)
\(Mg+2HCl\rightarrow MgCl_2+H_2\)
0,2-->0,4----->0,2------->0,2
a
\(V_{H_2}=0,2.22,4=4,48\left(l\right)\)
b
\(CM_{MgCl_2}=\dfrac{0,2}{0,2}=1M\)
c
\(MgCl_2+2NaOH\rightarrow Mg\left(OH\right)_2+2NaCl\)
0,2------>0,4
\(V_{dd.NaOH}=\dfrac{0,4}{2}=0,2\left(l\right)\)

\(\text{Đặt }n_{Al}=x(mol);n_{Fe}=y(mol)\\ \Rightarrow 27x+56y=13,9(1)\\ n_{H_2}=\dfrac{7,84}{22,4}=0,35(mol)\\ a,PTHH:2Al+6HCl\to 2AlCl_3+3H_2(1)\\ Fe+2HCl\to FeCl_2+H_2(2)\\ b,\text{Từ 2 PT: }1,5x+y=0,35(2)\\ (1)(2)\Rightarrow x=0,1(mol);y=0,2(mol)\\ \Rightarrow m_{Al}=0,1.27=2,7(g)\\ m_{Fe}=0,2.56=11,2(g)\)
\(c,n_{HCl(1)}=3n_{Al}=0,3(mol);n_{AlCl_3}=0,1(mol);n_{H_2(1)}=0,15(mol)\\ \Rightarrow m_{dd_{HCl(1)}}=\dfrac{0,3.36,5}{14,6\%}=75(g)\\ \Rightarrow C\%_{AlCl_3}=\dfrac{0,1.133,5}{2,7+75-0,15.2}.100\%=17,25\%\)
\(n_{HCl(2)}=2n_{Fe}=0,4(mol);n_{FeCl_2}=n_{H_2(2)}=n_{Fe}=0,2(mol)\\ \Rightarrow m{dd_{HCl(2)}}=\dfrac{0,4.36,5}{14,6\%}=100(g)\\ \Rightarrow C\%_{FeCl_2}=\dfrac{0,2.127}{11,2+100-0,2.2}.100\%=22,92\%\)
a) 2Al + 6HCl --> 2AlCl3 + 3H2
Fe + 2HCl --> FeCl2 + H2
b) Gọi số mol Al, Fe lần lượt là a,b
=> 27a + 56b = 13,9
\(n_{H_2}=\dfrac{7,84}{22,4}=0,35\left(mol\right)\)
2Al + 6HCl --> 2AlCl3 + 3H2
a----->3a--------->a------->1,5a______(mol)
Fe + 2HCl --> FeCl2 + H2
b------>2b-------->b----->b__________(mol)
=> 1,5a + b = 0,35
=> \(\left\{{}\begin{matrix}a=0,1=>m_{Al}=0,1.27=2,7\left(g\right)\\b=0,2=>m_{Fe}=0,2.56=11,2\left(g\right)\end{matrix}\right.\)
c) nHCl = 3a + 2b = 0,7 (mol)
=> mHCl = 0,7.36,5 = 25,55(g)
=> \(m_{ddHCl}=\dfrac{25,55.100}{14,6}=175\left(g\right)\)
\(m_{dd\left(saupu\right)}=13,9+175-2.0,35=188,2\left(g\right)\)
\(\left\{{}\begin{matrix}m_{AlCl_3}=0,1.133,5=13,35\left(g\right)\\m_{FeCl_2}=0,2.127=25,4\left(g\right)\end{matrix}\right.\)
=> \(\left\{{}\begin{matrix}C\%\left(AlCl_3\right)=\dfrac{13,35}{188,2}.100\%=7,1\%\\C\%\left(FeCl_2\right)=\dfrac{25,4}{188,2}.100\%=13,5\%\end{matrix}\right.\)

a, \(n_{H_2SO_4}=0,45.0,2=0,09\left(mol\right)\)
PTHH: FeO + H2SO4 → FeSO4 + H2O
Mol: a a
PTHH: MgO + H2SO4 → MgSO4 + H2O
Mol: b b
Ta có: \(\left\{{}\begin{matrix}72a+40b=4,48\\a+b=0,09\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,0275\\b=0,0625\end{matrix}\right.\)
\(\%m_{FeO}=\dfrac{0,0275.72.100\%}{4,48}=44,196\%\)
\(\%m_{MgO}=100-44,196=55,804\%\)
b,
PTHH: FeO + H2SO4 → FeSO4 + H2O
Mol: 0,0275 0,0275
PTHH: MgO + H2SO4 → MgSO4 + H2O
Mol: 0,0625 0,0625
\(C_{M_{ddFeSO_4}}=\dfrac{0,0275}{0,2}=0,1375M\)
\(C_{M_{ddMgSO_4}}=\dfrac{0,0625}{0,2}=0,3125M\)

Ta có: \(n_{H_2}=\dfrac{13,44}{22,4}=0,6\left(mol\right)\)
BTNT, có: \(n_{SO_4}=n_{H_2SO_4}=n_{H_2}=0,6\left(mol\right)\)
Mà: m muối = mKL + mSO4
⇒ m = mKL = 93,6 - 0,6.96 = 36 (g)
Bạn tham khảo nhé!

a, PT: \(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(Mg+2HCl\rightarrow MgCl_2+H_2\)
b, Gọi: \(\left\{{}\begin{matrix}n_{Fe}=x\left(mol\right)\\n_{Mg}=y\left(mol\right)\end{matrix}\right.\)
Theo PT: \(\left\{{}\begin{matrix}n_{HCl}=2n_{Fe}+2n_{Mg}=2x+2y\left(mol\right)\\n_{H_2}=n_{Fe}+n_{Mg}=x+y\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow m_{HCl}=36,5.\left(2x+2y\right)=73\left(x+y\right)\left(g\right)\)
\(\Rightarrow m_{ddHCl}=\dfrac{73\left(x+y\right)}{20\%}=365\left(x+y\right)\left(g\right)\)
Ta có: m dd sau pư = mFe + mMg + m dd HCl - mH2 = 56x + 24y + 365.(x+y) - 2.(x+y) = 419x + 387y (g)
Theo PT: \(n_{MgCl_2}=n_{Mg}=y\left(mol\right)\)
\(C\%_{MgCl_2}=11,87\%\) \(\Rightarrow\dfrac{95y}{419x+387y}=0,1187\)
\(\Rightarrow\dfrac{x}{y}=0,9865\Rightarrow x=0,9865y\)
Theo PT: \(n_{FeCl_2}=n_{Fe}=x\left(mol\right)\)
\(\Rightarrow C\%_{FeCl_2}=\dfrac{127x}{419x+387y}.100\%=\dfrac{127.0,9865y}{419.0,9865y+387y}.100\%\approx15,65\%\)

Bài 1:
PTHH: \(Mg+2HCl\rightarrow MgCl_2+H_2\uparrow\)
Ta có: \(n_{Mg}=\dfrac{4,8}{24}=0,2\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}n_{HCl}=0,4\left(mol\right)\\n_{H_2}=0,2\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}m_{ddHCl}=\dfrac{0,4\cdot36,5}{14,6\%}=100\left(g\right)\\V_{H_2}=0,2\cdot22,4=4,48\left(l\right)\end{matrix}\right.\)
Bài 2:
PTHH: \(2KOH+H_2SO_4\rightarrow K_2SO_4+2H_2O\)
Ta có: \(\left\{{}\begin{matrix}n_{KOH}=\dfrac{100\cdot11,2\%}{56}=0,2\left(mol\right)\\n_{H_2SO_4}=\dfrac{150\cdot9,8\%}{98}=0,15\left(mol\right)\end{matrix}\right.\)
Xét tỉ lệ: \(\dfrac{0,2}{2}< \dfrac{0,15}{1}\) \(\Rightarrow\) H2SO4 còn dư, KOH p/ứ hết
\(\Rightarrow\left\{{}\begin{matrix}n_{K_2SO_4}=0,1\left(mol\right)\\n_{H_2SO_4\left(dư\right)}=0,05\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}m_{K_2SO_4}=0,1\cdot174=17,4\left(g\right)\\m_{H_2SO_4\left(dư\right)}=0,05\cdot98=4,9\left(g\right)\end{matrix}\right.\)
Mặt khác: \(m_{dd}=m_{ddKOH}+m_{ddH_2SO_4}=250\left(g\right)\)
\(\Rightarrow\left\{{}\begin{matrix}C\%_{K_2SO_4}=\dfrac{17,4}{250}\cdot100\%=6,96\%\\C\%_{H_2SO_4\left(dư\right)}=\dfrac{4,9}{250}\cdot100\%=1,96\%\end{matrix}\right.\)
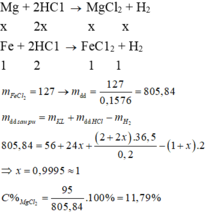
Bài 1:
PTHH: \(Mg+2HCl\rightarrow MgCl_2+H_2\uparrow\)
Ta có: \(n_{Mg}=\dfrac{1}{2}n_{HCl}=\dfrac{1}{2}\cdot0,11\cdot1,5=0,0825\left(mol\right)\)
\(\Rightarrow m_{Mg}=0,0825\cdot24=1,98\left(g\right)\)
Bài 3:
Vì Cu không tác dụng với nước
\(\Rightarrow m=m_{Cu}=0,1\cdot64=6,4\left(g\right)\)