Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


\(13,n_{Fe}=\dfrac{16,8}{56}=0,3\left(mol\right)\\ PTHH:Fe+2HCl\rightarrow FeCl_2+H_2\\ .....0,3.....0,6......0,3......0,3\left(mol\right)\\ V_{H_2\left(đktc\right)}=0,3\cdot22,4=6,72\left(l\right)\\ 14,n_{CaCO_3}=\dfrac{25}{40+12+16\cdot3}=0,25\left(mol\right)\\ PTHH:CaCO_3+2HCl\rightarrow CaCl_2+H_2O+CO_2\\ .....0,25.....0,5......0,25......0,25......0,25\left(mol\right)\\ V_{CO_2\left(đktc\right)}=0,25\cdot22,4=5,6\left(l\right)\)

a, Ta có: \(n_{Zn}=\dfrac{6,5}{65}=0,1\left(mol\right)\)
PT: \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
___0,1_________________0,1 (mol)
Ta có: \(V_{H_2}=0,1.22,4=2,24\left(l\right)\)
b, PT: \(Fe_2O_3+3H_2\underrightarrow{t^o}2Fe+3H_2O\)
Theo PT: \(n_{Fe}=\dfrac{2}{3}n_{H_2}=\dfrac{1}{15}\left(mol\right)\)
\(\Rightarrow m_{Fe}=\dfrac{1}{15}.56\approx3,73\left(g\right)\)
Bạn tham khảo nhé!

F e + 2 H C l → F e C L 2 + H 2
Vì H = 85% nên:
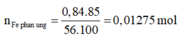
n H 2 = n F e p ư = 0,01275 mol
V H 2 = 0,01275.22,4= 0,2856 lit
⇒ Chọn C.

a) \(n_{HCl}=0,4.1=0,4\left(mol\right)\)
PTHH: Fe + 2HCl → FeCl2 + H2
Mol: 0,2 0,4 0,2
b, \(V_{H_2}=0,2.22,4=4,48\left(l\right)\)
\(m_{Fe}=0,2.56=11,2\left(g\right)\)
c, \(n_{Cu\left(tt\right)}=\dfrac{10,24}{64}=0,16\left(mol\right)\)
PTHH: H2 + CuO → Cu + H2O
Mol: 0,2 0,2
\(\Rightarrow H=\dfrac{n_{Cu\left(tt\right)}}{n_{Cu\left(lt\right)}}=\dfrac{0,16}{0,2}.100\%=80\%\)

a) \(n_{Fe}=\dfrac{m_{Fe}}{M_{Fe}}=\dfrac{28}{56}=0,5\left(mol\right)\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
0,5-------1---------0,5------0,5
b) \(V_{H_2}=n_{H_2}.22,4=0,5.22,4=11,2\left(l\right)\)
c) \(H_2+CuO\rightarrow Cu+H_2O\)
0,5-----0,5------0,5----0,5
Khối lượng đồng tạo thành: \(m_{Cu}=n_{Cu}.64=0,5.64=32\left(g\right)\)

a) \(n_{Fe}=\dfrac{28}{56}=0,5\left(mol\right)\)
PTHH: `Fe + 2HCl -> FeCl_2 + H_2`
0,5-------------------------->0,5`
b) `V_{H_2} = 0,5.22,4 = 11,2 (l)`
c) PTHH: \(CuO+H_2\xrightarrow[]{t^o}Cu+H_2O\)
0,5---->0,5
`=> m_{Cu} = 0,5.64 = 32 (g)`
\(Fe+2HCl\underrightarrow{t^o}FeCl_2+H_2\)
\(1mol\) \(1mol\)
\(0,5mol\) \(0,5mol\)
\(n_{Fe}=\dfrac{m}{M}=\dfrac{28}{56}=0,5\left(mol\right)\)
\(V_{H_2}=n.22,4=0,5.22,4=11,2\left(l\right)\)
\(H_2+CuO\underrightarrow{t^o}Cu+H_2O\)
\(1mol\) \(1mol\)
\(0,5mol\) \(0,5mol\)
\(m_{Cu}=n.M=0,5.64=32\left(g\right)\)

\(n_{Zn}=\dfrac{16,25}{65}=0,25\left(mol\right)\\ Zn+2HCl\rightarrow ZnCl_2+H_2\\ n_{H_2}=n_{Zn}=0,25\left(mol\right)\\ V_{H_2\left(đktc\right)}=0,25.22,4=5,6\left(l\right)\)
Zn + 2HCl -> ZnCl2 + H2
0.25 0.25
nZn = 0.25 mol
\(V_{H2}=0.25\times22.4=5.6l\)

a, \(MnO_2+4HCl\rightarrow MnCl_2+Cl_2+2H_2O\)
Ta có: \(n_{MnO_2}=\dfrac{34,8}{87}=0,4\left(mol\right)\)
Theo PT: \(n_{Cl_2}=n_{MnO_2}=0,4\left(mol\right)\Rightarrow V_{Cl_2}=0,4.22,4=8,96\left(l\right)\)
b, \(n_{H_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\)
PT: \(Cl_2+H_2\underrightarrow{as}2HCl\)
Xét tỉ lệ: \(\dfrac{0,4}{1}< \dfrac{0,5}{1}\), ta được H2 dư.
Theo PT: \(\left\{{}\begin{matrix}n_{H_2\left(pư\right)}=n_{Cl_2}=0,4\left(mol\right)\\n_{HCl}=2n_{Cl_2}=0,8\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow n_{H_2\left(dư\right)}=0,5-0,4=0,1\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}\%V_{HCl}=\dfrac{0,8.22,4}{\left(0,8+0,1\right).22,4}.100\%\approx88,89\%\\\%V_{H_2\left(dư\right)}\approx11,11\%\end{matrix}\right.\)
c, Ta có: \(m_{HCl}=0,8.36,5=29,2\left(g\right)\)
\(\Rightarrow C\%_{HCl}=\dfrac{29,2}{200}.100\%=14,6\%\)
d, \(n_{NaOH}=0,5.4=2\left(mol\right)\)
PT: \(Cl_2+2NaOH\rightarrow NaCl+NaClO+H_2O\)
Xét tỉ lệ: \(\dfrac{0,4}{1}< \dfrac{2}{2}\), ta được NaOH dư.
Theo PT: \(\left\{{}\begin{matrix}n_{NaCl}=n_{NaClO}=n_{Cl_2}=0,4\left(mol\right)\\n_{NaOH\left(pư\right)}=2n_{Cl_2}=0,8\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow n_{NaOH\left(dư\right)}=2-0,8=1,2\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}C_{M_{NaCl}}=C_{M_{NaClO}}=\dfrac{0,4}{0,5}=0,8\left(M\right)\\C_{M_{NaOH\left(dư\right)}}=\dfrac{1,2}{0,5}=2,4\left(M\right)\end{matrix}\right.\)

nFe = 11,2/56 = 0,2 (mol)
nHCl = 18,25/36,5 = 0,5 (mol)
PTHH: Fe + 2HCl -> FeCl2 + H2
LTL: 0,2 < 0,5/2 => HCl dư
nH2 (lt) = nFe = 0,2 (mol)
VH2 (lt) = 0,2 . 22,4 = 4,48 (l)
VH2 (tt) = 4,48 . 60% = 2,688 (l)
\(Fe + 2HCl \to FeCl_2 + H_2\\ n_{H_2} = n_{Fe} = \dfrac{12}{56} = \dfrac{3}{14}(mol)\\ \Rightarrow V_{H_2} = \dfrac{3}{14}.22,4 = 4,8(lít)\)
Bạn ơi chắc này là câu cuối trong bài toán của hóa nhỉ? Nếu vậy thì bạn cần cung cấp đủ đề bài thì mới làm được!