Trộn 100 gam dung dịch H2SO4 19,6% với 300 gam dung dịch BaCl2 20,8% thu được m gam kết tủa
và dung dịch Y.
1.Viết phương trình phản ứng? Tính m?
2.Tính nồng độ % của mỗi chất trong dung dịch Y?
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(a,PTHH:H_2SO_4+BaCl_2\rightarrow BaSO_4\downarrow+2HCl\\ \left\{{}\begin{matrix}m_{H_2SO_4}=\dfrac{300\cdot9,8\%}{100\%}=29,4\left(g\right)\\m_{BaCl_2}=\dfrac{200\cdot26\%}{100\%}=52\left(g\right)\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}n_{H_2SO_4}=\dfrac{29,4}{98}=0,3\left(mol\right)\\n_{BaCl_2}=\dfrac{52}{208}=0,25\left(mol\right)\end{matrix}\right.\)
Vì \(\dfrac{n_{H_2SO_4}}{1}>\dfrac{n_{BaCl_2}}{1}\) nên H2SO4 dư
\(\Rightarrow n_{BaSO_4}=n_{BaCl_2}=0,25\left(mol\right)\\ \Rightarrow a=m_{BaSO_4}=0,25\cdot233=58,25\left(g\right)\\ b,n_{HCl}=n_{BaCl_2}=0,25\left(mol\right)\\ \Rightarrow m_{CT_{HCl}}=0,25\cdot36,5=9,125\left(g\right)\\ m_{dd_{HCl}}=300+200-58,25=441,75\left(g\right)\\ \Rightarrow C\%_{HCl}=\dfrac{9,125}{441,75}\cdot100\%\approx2,07\%\)

a) Vì: mA < 400 (g) nên phải có khí thoát ra → muối có dạng MHSO4 và khí là: CO2
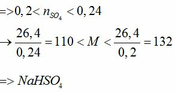
b)
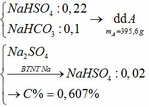
c) Tác dụng được với: MgCO3, Ba(HSO3)2, Al2O3, Fe(OH)2, Fe, Fe(NO3)2
Pt: 2NaHSO4 + MgCO3 → Na2SO4 + MgSO4 + CO2↑ + H2O
2NaHSO4 + Ba(HSO3)2 → BaSO4 + Na2SO4 + SO2↑ + 2H2O
6NaHSO4 + Al2O3 → 3Na2SO4 + Al2(SO4)3 + 3H2O
2NaHSO4 + Fe(OH)2 → Na2SO4 + FeSO4 + 2H2O
2NaHSO4 + Fe → Na2SO4 + FeSO4 + H2↑
12NaHSO4 + 9Fe(NO3)2 → 5Fe(NO3)3 + 2Fe2(SO4)3 + 6Na2SO4 + 3NO↑ + 6H2O

\(n_{NaOH}=1.0,4=0,4(mol);n_{FeCl_3}=1.0,1=0,1(mol)\\ a,PTHH:3NaOH+FeCl_3\to Fe(OH)_3\downarrow+3NaCl\\ \text {Vì }\dfrac{n_{NaOH}}{3}>\dfrac{n_{FeCl_3}}{1} \text {nên }NaOH\text { dư}\\ \Rightarrow n_{Fe(OH)_3}=0,1(mol)\\ \Rightarrow m_{Fe(OH)_3}=107.0,1=10,7(g)\\ b,n_{NaCl}=3n_{FeCl_3}=0,3(mol)\\ \Rightarrow C_{M_{NaCl}}=\dfrac{0,3}{0,4+0,1}=0,6M\)

Bài 19 :
\(a) n_{Al} = \dfrac{10,8}{27} = 0,4(mol)\\ 2Al + 3H_2SO_4 \to Al_2(SO_4)_3 + 3H_2\\ n_{H_2} = \dfrac{3}{2}n_{Al} = 0,6(mol)\\ V_{H_2} = 0,6.22,4 = 13,44(lít)\\ b) \text{Chất tan : }Al_2(SO_4)_3\\ n_{Al_2(SO_4)_3} = \dfrac{1}{2}n_{Al} = 0,2(mol)\\ m_{Al_2(SO_4)_3} = 0,2.342 = 68,4(gam)\)
Bài 18 :
\(a) n_{HCl} = \dfrac{250.7,3\%}{36,5 } = 0,5(mol)\\ Zn + 2HCl \to ZnCl_2 + H_2\\ n_{H_2} = \dfrac{1}{2}n_{HCl} = 0,25(mol) \Rightarrow V_{H_2} = 0,25.22,4 = 5,6(lít)\\ b) \text{Chất tan : } ZnCl_2\\ n_{ZnCl_2} = n_{H_2} = 0,25(mol)\\ m_{ZnCl_2} = 0,25.136 = 34(gam)\)

\(a,\left\{{}\begin{matrix}m_{H_2SO_4}=\dfrac{300\cdot9,8\%}{100\%}=29,4\left(g\right)\\m_{BaCl_2}=\dfrac{200\cdot26\%}{100\%}=52\left(g\right)\end{matrix}\right.\\ \Rightarrow\left\{{}\begin{matrix}n_{H_2SO_4}=\dfrac{29,4}{98}=0,3\left(mol\right)\\n_{BaCl_2}=\dfrac{52}{208}=0,25\left(mol\right)\end{matrix}\right.\\ PTHH:H_2SO_4+BaCl_2\rightarrow2HCl+BaSO_4\downarrow\)
Vì \(\dfrac{n_{H_2SO_4}}{1}>\dfrac{n_{BaCl_2}}{1}\) nên sau phản ứng \(H_2SO_4\) dư
\(\Rightarrow n_{BaSO_4}=0,25\left(mol\right)\\ \Rightarrow a=m_{BaSO_4}=0,25\cdot233=58,25\left(g\right)\)
\(b,n_{HCl}=2n_{BaCl_2}=0,5\left(mol\right)\\ \Rightarrow m_{CT_{HCl}}=0,5\cdot36,5=18,25\left(g\right)\\ m_{dd_{HCl}}=300+200-58,25=441,75\left(g\right)\\ \Rightarrow C\%_{HCl}=\dfrac{18,25}{441,75}\cdot100\%\approx4,13\%\)

\(n_{KOH}=\dfrac{400.7\%}{56}=0,5\left(mol\right)\)
\(n_{H_2SO_4}=\dfrac{100.19,6\%}{98}=0,2\left(mol\right)\)
PTHH: 2KOH + H2SO4 --> K2SO4 + 2H2O
Xét tỉ lệ: \(\dfrac{0,5}{2}>\dfrac{0,2}{1}\) => KOH dư, H2SO4 hết
PTHH: 2KOH + H2SO4 --> K2SO4 + 2H2O
0,4<----0,2-------->0,2
=> \(\left\{{}\begin{matrix}m_{KOH\left(dư\right)}=\left(0,5-0,4\right).56=5,6\left(g\right)\\m_{K_2SO_4}=0,2.174=34,8\left(g\right)\end{matrix}\right.\)
mdd sau pư = 400 + 100 = 500 (g)
=> \(\left\{{}\begin{matrix}C\%_{KOH.dư}=\dfrac{5,6}{500}.100\%=1,12\%\\C\%_{K_2SO_4}=\dfrac{34,8}{500}.100\%=6,96\%\end{matrix}\right.\)

\(n_{KOH}=\dfrac{400.7}{100}:56=0,5\left(mol\right)\)
\(n_{H_2SO_4}=\dfrac{100.19,6}{100}:98=0,2\left(mol\right)\)
\(2KOH+H_2SO_4\rightarrow K_2SO_4+2H_2O\)
0,4 0,2 0,2
Lập tỉ lệ:
\(\dfrac{0,5}{2}>\dfrac{0,2}{1}\) => KOH dư.
\(m_{dd}=400+100=500\left(g\right)\)
\(n_{KOH.dư}=0,5-0,4=0,1\left(mol\right)\)
\(C\%_{K_2SO_4}=\dfrac{0,2.174.100}{500}=6,96\%\)
\(C\%_{KOH}=\dfrac{0,1.56.100}{500}=1,12\%\)

a, \(BaCl_2+H_2SO_4\rightarrow2HCl+BaSO_{4\downarrow}\)
b, \(m_{BaCl_2}=200.2,08\%=4,16\left(g\right)\Rightarrow n_{BaCl_2}=\dfrac{4,16}{208}=0,02\left(mol\right)\)
\(m_{H_2SO_4}=300.9,8\%=29,4\left(g\right)\Rightarrow n_{H_2SO_4}=\dfrac{29,4}{98}=0,3\left(mol\right)\)
Xét tỉ lệ: \(\dfrac{0,02}{1}< \dfrac{0,3}{1}\), ta được H2SO4 dư.
Theo PT: \(\left\{{}\begin{matrix}n_{BaSO_4}=n_{H_2SO_4\left(pư\right)}=n_{BaCl_2}=0,02\left(mol\right)\\n_{HCl}=2n_{BaCl_2}=0,04\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow n_{H_2SO_4\left(dư\right)}=0,3-0,02=0,28\left(mol\right)\)
Ta có: m dd sau pư = m dd BaCl2 + m dd H2SO4 - mBaSO4 = 200 + 300 - 0,02.233 = 495,34 (g)
\(\Rightarrow\left\{{}\begin{matrix}C\%_{HCl}=\dfrac{0,04.36,5}{495,34}.100\%\approx0,295\%\\C\%_{H_2SO_4\left(dư\right)}=\dfrac{0,28.98}{495,34}.100\%\approx5,54\%\end{matrix}\right.\)

a
PTHH của phản ứng xảy ra:
\(Na_2SO_4+BaCl_2\rightarrow BaSO_4\downarrow+2NaCl\)
b
\(n_{Na_2SO_4}=0,1.0,5=0,05\left(mol\right)\)
\(\Rightarrow n_{BaSO_4}=n_{Na_2SO_4}=0,05\left(mol\right)\) (dựa theo PTHH)
\(\Rightarrow m_{\downarrow}=m_{BaSO_4}=233.0,05=11,65\left(g\right)\)
c
Theo PTHH có: \(n_{BaCl_2\left(đã.dùng\right)}=n_{Na_2SO_4}=0,05\left(mol\right)\)
\(\Rightarrow CM_{BaCl_2}=\dfrac{n}{V}=\dfrac{0,05}{50:1000}=1M\)
\(\begin{cases} m_{H_2SO_4}=\dfrac{100.19,6\%}{100\%}=19,6(g)\\ m_{BaCl_2}=\dfrac{300.20,8\%}{100\%}=62,4(g) \end{cases} \Rightarrow \begin{cases} n_{H_2SO_4}=\dfrac{19,6}{98}=0,2(mol)\\ n_{BaCl_2}=\dfrac{62,4}{208}=0,3(mol) \end{cases}\\ a,PTHH:BaCl_2+H_2SO_4\to BaSO_4\downarrow +2HCl\)
Vì \(\dfrac{n_{H_2SO_4}}{1}<\dfrac{n_{BaCl_2}}{1}\) nên \(BaCl_2\) dư
\(\Rightarrow n_{BaSO_4}=0,2(mol)\\ \Rightarrow m_{BaSO_4}=0,2.233=46,6(g)\)
\(b,n_{HCl}=n_{BaSO_4}=0,2(mol)\\ \Rightarrow m_{CT_{HCl}}=0,2.36,5=7,3(g)\\ m_{dd_{HCl}}=100+300-46,6=353,4(g)\\ \Rightarrow C\%_{HCl}=\dfrac{7,3}{353,4}.100\%\approx 2,07\%\)