Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Fe+2HCl--->FeCl2 +H2
x-----------------x
MgO +2HCl----->MgCl2 +H2
y------------------------y
Ta có
\(\left\{{}\begin{matrix}56x+40y=13,6\\127x+95y=31,7\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}x=0,1\\y=0,2\end{matrix}\right.\)
%m\(_{Fe}=\frac{0,1.56}{13,6}.100\%=41,18\%\)
%m\(_{MgO}=100-41,18=58,52\left(g\right)\)
Chúc bạn học tốt

Gọi \(\left\{{}\begin{matrix}n_{CuO}=x\\n_{MgO}=y\end{matrix}\right.\)
\(CuO+2HCl\rightarrow CuCl_2+H_2O\)
x x ( mol )
\(MgO+2HCl\rightarrow MgCl_2+H_2O\)
y y ( mol )
Ta có:
\(\left\{{}\begin{matrix}80x+40y=16\\135x+95y=32,5\end{matrix}\right.\) \(\Leftrightarrow\left\{{}\begin{matrix}x=0,1\\y=0,2\end{matrix}\right.\)
\(\Rightarrow m_{CuO}=0,1.80=8g\)
\(\Rightarrow m_{MgO}=0,2.40=8g\)
\(\%m_{CuO}=\dfrac{8}{16}.100=50\%\)
\(\%m_{MgO}=\dfrac{8}{16}.100=50\%\)
\(m_{CuCl_2}=0,1.135=13,5g\)
\(m_{MgCl_2}=0,2.95=19g\)

\(n_{H_2}=\dfrac{7,84}{22,4}=0,35mol\)
Gọi \(\left\{{}\begin{matrix}n_{Fe}=x\\n_{Zn}=y\end{matrix}\right.\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
x x ( mol )
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
y y ( mol )
Ta có:
\(\left\{{}\begin{matrix}56x+65y=21,4\\x+y=0,35\end{matrix}\right.\) \(\Leftrightarrow\left\{{}\begin{matrix}x=0,15\\y=0,2\end{matrix}\right.\)
\(\Rightarrow m_{Fe}=0,15.56=8,4g\)
\(\Rightarrow m_{Zn}=0,2.65=13g\)
\(\%m_{Fe}=\dfrac{8,4}{21,4}.100=39,25\%\)
\(\%m_{Zn}=100\%-39,25\%=60,75\%\)
\(m_{FeCl_2}=0,15.127=19,05g\)
\(m_{ZnCl_2}=0,2.136=27,2g\)

nH2 \(\approx\)0,2 (mol)
Mg + 2HCl \(\rightarrow\) MgCl2 + H2 (1)
0,2 <------------ 0,2 <----- 0,2 (mol)
MgO + 2HCl \(\rightarrow\) MgCl2 + H2O (2)
b) %mMg = \(\frac{0,2.24}{8,8}\) . 100% =54,55%
%mMgO = 45,45%
c) mMgO = 8,8 - 0,2 . 24 = 4(g)
=> nMgO=0,1 (mol)
Theo pt(2) nMgCl2 = nMg = 0,1 (mol)
=> \(\Sigma n_{MgCl_2}\) = 0,2 + 0,1 = 0,3 (mol)
mmuối = 0,3 . 95 = 28,5 (g)

\(Đặt:n_{MnO_2}=a\left(mol\right),n_{KMnO_4}=b\left(mol\right)\)
\(m_{hh}=87a+158b=37.96\left(g\right)\left(1\right)\)
\(n_{Cl_2}=\dfrac{10.08}{22.4}=0.45\left(mol\right)\)
\(2KMnO_4+16HCl\rightarrow2KCl+2MnCl_2+5Cl_2+8H_2O\)
\(MnO_2+4HCl\rightarrow MnCl_2+Cl_2+2H_2O\)
\(n_{Cl_2}=a+2.5b=0.45\left(mol\right)\left(2\right)\)
\(\left(1\right),\left(2\right):a=0.4,b=0.02\)
\(\%MnO_2=\dfrac{0.4\cdot87}{37.96}\cdot100\%=91.68\%\\\%KMnO_4=100-91.68=8.32\% \)
\(m_M=m_{KCl}+m_{MnCl_2}=0.02\cdot74.5+\left(0.4+0.02\right)\cdot126=54.41g\)

a) Gọi x,y lần lượt là số mol Fe, Cu trong hhX (x,y>0) (mol)
- Khi cho X t/d hoàn toàn với khí Clo dư:
\(2Fe+3Cl_2\rightarrow\left(t^o\right)2FeCl_3\\ Cu+Cl_2\rightarrow\left(t^o\right)CuCl_2\\ \Rightarrow162,5x+135y=59,5\left(1\right)\)
- Khi cho X tác dụng hoàn toàn với dd HCl 36,5%. Cu sẽ không tác dụng mà chỉ có Fe tham gia phản ứng.
\(Fe+2HCl\rightarrow FeCl_2+H_2\\ m_{FeCl_2}=127x=25,4\left(g\right)\left(2\right)\\ \left(1\right),\left(2\right)\Rightarrow\left\{{}\begin{matrix}162,5x+135y=59,5\\127x=25,4\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}x=0,2\\y=0,2\end{matrix}\right.\\ \Rightarrow a=m_{hhX}=m_{Fe}+m_{Cu}=64x+56y=64.0,2+56.0,2=24\left(g\right)\)
Tính phần trăm mỗi muối sau phản ứng chắc ở phản ứng với Clo dư.
\(\%m_{FeCl_3}=\dfrac{0,2.162,5}{0,2.162,5+0,2.135}.100\approx54,622\%\\ \Rightarrow\%m_{CuCl_2}\approx45,378\%\)
b)
\(n_{HCl}=2x=2.0,2=0,4\left(mol\right)\\ \Rightarrow m_{HCl}=0,4.36,5=14,6\left(g\right)\\ \Rightarrow m_{ddHCl}=\dfrac{14,6.100}{36,5}=40\left(g\right)\\ \Rightarrow V_{ddHCl}=\dfrac{m_{ddHCl}}{D_{ddHCl}}=\dfrac{40}{1,25}=32\left(ml\right)=0,032\left(l\right)\)




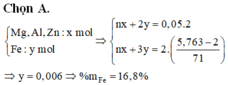
Đặt :
nFe = x mol
nMgO = y mol
mX = 56x + 40y = 13.6 (g) (1)
Fe + 2HCl => FeCl2 + H2
x____________x
MgO + 2HCl => MgCl2 + H2O
y______________y
mM = mFeCl2 + mMgCl2 = 127x + 95y = 31.7 (2)
(1) , (2) :
x = 0.1
y = 0.2
%Fe = 5.6/13.6 * 100% = 41.17%
%MgO = 58.82%
nKOH = 0.1 * 0.2 = 0.02 (mol)
KOH + HCl => KCl + H2O
0.02____0.02
nHCl (pư) = 2nFe + 2nMgO = 0.1*2 + 0.2*2 = 0.6 (mol)
nHCl = 0.02 + 0.6 = 0.62 (mol)
VddHCl = 0.62/0.5 = 1.24 (M)