Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(m_{H_2}=0,01a\left(g\right)\)
=> \(n_{H_2}=\dfrac{0,01a}{2}=0,005a\left(mol\right)\)
PTHH: Fe + 2HCl --> FeCl2 + H2
0,005a<----------------0,005a
=> mFe = 56.0,005a = 0,28a (g)
Gọi số mol FeO, Fe2O3 là x, y (mol)
=> 72x + 160y = a - 0,28a = 0,72a (1)
\(m_{H_2O}=0,2115a\left(g\right)\)
=> \(n_{H_2O}=\dfrac{0,2115a}{18}=0,01175a\left(mol\right)\)
PTHH: FeO + H2 --to--> Fe + H2O
x---------------------->x
Fe2O3 + 3H2 --to--> 2Fe + 3H2O
y----------------------------->3y
=> x + 3y = 0,01175a (2)
(1)(2) => \(\left\{{}\begin{matrix}x=0,005a\left(mol\right)\\y=0,00225a\left(mol\right)\end{matrix}\right.\)
=> \(\%Fe=\dfrac{0,28a}{a}.100\%=28\%\)
\(\%FeO=\dfrac{72.0,005a}{a}.100\%=36\%\)
\(\%Fe_2O_3=\dfrac{160.0,00225a}{a}.100\%=36\%\)
\(m_{H_2}=0,01a\left(g\right)\\ \Rightarrow n_{Fe}=n_{H_2}=0,005a\left(mol\right)\\\Rightarrow m_{FeO,Fe_2O_3}=a-0,005a.56=0,72a\\ Đặt:n_{FeO}=x\left(mol\right);n_{Fe_2O_3}=y\left(mol\right)\left(x,y>0\right)\\ \Rightarrow72x+160y=0,72a\left(1\right)\\ m_{H_2O}=0,2115a\\ \Leftrightarrow18x+54y=0,2115a\left(2\right)\\ \left(1\right),\left(2\right)\Rightarrow\dfrac{504}{47}x=\dfrac{1120}{47}y\\ \Rightarrow\dfrac{x}{y}=\dfrac{\dfrac{1120}{47}}{\dfrac{504}{47}}=\dfrac{20}{9}\\ \Rightarrow\%m_{Fe}=\dfrac{0,28a}{a}.100=28\%\\Ta.có:x.72+0,45x.160=0,72a\\ \Leftrightarrow144x=0,72a\\ \Leftrightarrow\dfrac{x}{a}=\dfrac{0,72}{144}=0,005\\ \Rightarrow\%m_{FeO}=\dfrac{72.0,005a}{a}.100=36\%\)
\(\Rightarrow\%m_{Fe_2O_3}=100\%-\left(28\%+36\%\right)=36\%\)

Cau 1 :
\(n_{H2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
Pt : \(Fe+2HCl\rightarrow FeCl_2+H_2|\)
1 2 1 1
0,1 0,1
a) Chat trong dung dich A thu duoc la : sat (II) clorua
Chat ran B la : dong
Chat khi C la : khi hidro
b) \(n_{Fe}=\dfrac{0,1.1}{1}=0,1\left(mol\right)\)
\(m_{Fe}=0,1.56=5,6\left(g\right)\)
\(m_{Cu}=10-5,6=4,4\left(g\right)\)
0/0Fe = \(\dfrac{5,6.100}{10}=56\)0/0
0/0Cu = \(\dfrac{4,4.100}{10}=44\)0/0
c) Co : \(m_{Cu}=4,4\left(g\right)\)
\(n_{Cu}=\dfrac{4,4}{64}=0,06875\left(mol\right)\)
Pt : \(Cu+2H_2SO_{4dac}\underrightarrow{t^o}CuSO_4+SO_2+2H_2O|\)
1 2 1 1 2
0,06875 0,06875
\(n_{SO2}=\dfrac{0,06875.1}{1}=0,06875\left(mol\right)\)
\(V_{SO2\left(dktc\right)}=1,54\left(l\right)\)
Chuc ban hoc tot
Minh xin loi ban nhe , ban bo sung vao cho :
\(V_{SO2\left(dktc\right)}=0,06875.22,4=1,54\left(l\right)\)

a)
Gọi số mol Al2O3 và ZnO là a,b
=> 102a + 81b = 26,4
nHNO3 = 0,2.5 = 1 (mol)
PTHH: Al2O3 + 6HNO3 --> 2Al(NO3)3 + 3H2O
_______a----->6a----------->2a
ZnO + 2HNO3 --> Zn(NO3)2 + H2O
_b---->2b---------->b
=> 6a + 2b = 1
=> a = 0,1 ; b = 0,2
=> \(\left\{{}\begin{matrix}m_{Al_2O_3}=0,1.102=10,2\left(g\right)\\m_{ZnO}=0,2.81=16,2\left(g\right)\end{matrix}\right.\)
=> \(\left\{{}\begin{matrix}\%Al_2O_3=\dfrac{10,2}{26,4}.100\%=38,636\%\\\%ZnO=\dfrac{16,2}{26,4}.100\%=61,364\%\end{matrix}\right.\)
b)
\(\left\{{}\begin{matrix}n_{Al\left(NO_3\right)_3}=0,2\left(mol\right)\\n_{Zn\left(NO_3\right)_2}=0,2\left(mol\right)\end{matrix}\right.\)
PTHH: 4Al(NO3)3 --to--> 2Al2O3 + 12NO2 + 3O2
________0,2------------->0,1
2Zn(NO3)2 --to--> 2ZnO + 4NO2 + O2
__0,2------------->0,2
=> mrắn = 0,1.102 + 0,2.81 = 26,4 (g)

80 gam dung dịch A chứa 3,52 gam NaOH
=> 200 gam dung dịch A chứa 3,52.200/80 = 8,8 gam
n NaOH = 8,8/40 = 0,22(mol)
Gọi n Na = a(mol) ; n Na2O = b(mol)
=> 23a + 62b = 6,02(1)
$2Na + 2H_2O \to 2NaOH + H_2$
$Na_2O + H_2O \to 2NaOH$
n NaOH = a + 2b = 0,22(2)
Từ (1)(2) suy ra a= 0,1 ; b = 0,06
n H2 = 0,5a = 0,05(mol)
=> m H2O = 200 + 0,05.2 - 6,02 =194,08(gam)
%m Na = 0,1.23/6,02 .100% = 38,2%
%m Na2O = 100% -38,2% = 61,8%

PTHH:
\(CaCO_3+2HCl\rightarrow CaCl_2+H_2O+CO_2\uparrow\)
0,15 0,15
\(CaO+2HCl\rightarrow CaCl_2+H_2O\)
Ta có: \(n_{CO_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
\(\Rightarrow m_{CaCO_3}=0,15\cdot100=15\left(g\right)\)
\(\Rightarrow m_{CaO}=20,6-15=5,6\left(g\right)\)
\(\Rightarrow\%m_{CaCO_3}=\dfrac{15\cdot100}{20,6}\approx73\%\)
\(\Rightarrow\%m_{CaO}=100\%-73\%=27\%\)

\(Fe+2HCl\rightarrow FeCl_2+H_2\)
Chất rắn không tan : Cu
\(m_{Cu}=3.2\left(g\right)\Rightarrow m_{Fe}=8-3.2=4.8\left(g\right)\)
\(\%Fe=\dfrac{4.8}{8}\cdot100\%=60\%\)
\(\%Cu=100\%-60\%=40\%\)
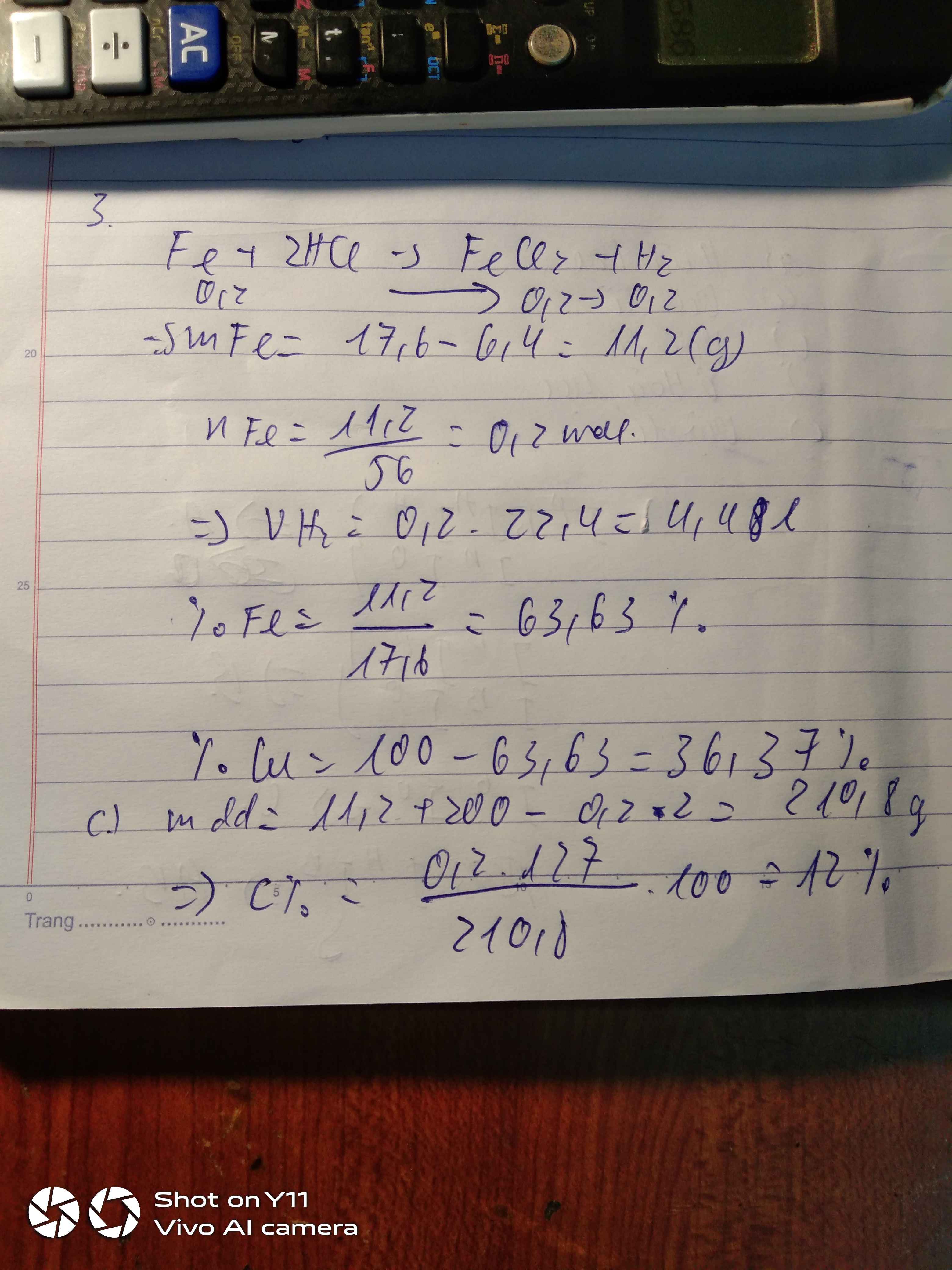
a) PTHH: \(Fe+2HCl\rightarrow FeCl_2+H_2\uparrow\)
b) Theo bài ra, ta có: \(m_{Cu}=3,2\left(g\right)\)
\(\Rightarrow\%m_{Cu}=\dfrac{3,2}{12}\cdot100\%\approx26,67\%\) \(\Rightarrow\%m_{Fe}=73,33\%\)
c) Ta có: \(n_{Fe}=\dfrac{12-3,2}{56}=\dfrac{11}{70}\left(mol\right)=m_{FeCl_2}\)
\(\Rightarrow m_{FeCl_2}=\dfrac{11}{70}\cdot127\approx19,96\left(g\right)\)
a) PTHH: Fe+2HCl→FeCl2+H2↑
b) Theo bài ra, ta có: mCu=3,2(g)
⇒%mCu=3,212⋅100%≈26,67% ⇒%mFe=73,33%
c) Ta có: nFe=12−3,256=1170(mol)=mFeCl2
⇒mFeCl2=1170⋅127≈19,96(g)