 mọi người làm bài 2 bài 3 giúp em với ạ (em cảm ơn)
mọi người làm bài 2 bài 3 giúp em với ạ (em cảm ơn)
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


a) Ta có: AB//CD.
=>ABH=BDC (2 góc so le trong).
=> ∆AHB~∆BCD(g.g).
b) ∆ABD có : DB²=AB²+AD²( Định lý Pitago)
=> DB= 15(cm).
Ta có ∆ABH~∆BCD(cmt).
=>AH/BC=AD/BD.
Hay AH=9.12/15=7,2(cm).
c)Ta có ∆AHB~∆BCD cmt.
=> HBA=CBD. (1)
Ta lại có : CBD= ADH (AB//CD).(2)
Từ 1 và 2 => HAB=ADH.
=>∆DHA~∆AHB(g.g).
S∆DHA/S∆AHB=(AD/AB)²=9/16
d) từ câu (a) và (b) => ∆BCD~∆DHA.
Cm ∆DHA~∆MDA(g.g)
Từ đó suy ra ∆BDC~∆MDA.
Sau đó cm ∆BCD~∆ADC(g.g).
=> ∆MDA~∆ADC(g.g).
=>Ad/DC=DM/DC.
=>Đpcm.

1. English is more interesting than music.
2. Today they are not as happy as they were yesterday.
3. Ha Noi is not as small as Hai Duong.
4. Mai's sister is not as pretty as her.
6. You have got more money than me.
7. Art is not as difficult as French.
8. Nam's father is more careful than him.
9. No one in our town is as rich as Mr Ron.
10. He is the most intelligent in my class.
11. Everest is the highest mountain in the world.
12. Minh is the fattest person in my group.
13. I can't swim as far as Jan.
14B 15C 16A 17C 18B 19C 20B

Câu 6:
Gọi kim loại đó là \(R\)
\(\rightarrow Oxit:R_2O_3\)
Giả sử dd \(H_2SO_4\) phản ứng \(a\left(mol\right)\)
\(PTHH:R_2O_3+3H_2SO_4\rightarrow R_2\left(SO_4\right)_3+3H_2O\)
\(\left(mol\right)\) \(\dfrac{a}{3}\) \(a\) \(\dfrac{a}{3}\)
\(m_{ddH_2SO_4}=\dfrac{98a.100}{10}=980a\left(g\right)\)
\(C\%_{ddspu}=12,9\left(\%\right)\Leftrightarrow\dfrac{\left(2R+288\right).\dfrac{a}{3}}{\left(2R+48\right).\dfrac{a}{3}+980a}.100=12,9\\ \Leftrightarrow\dfrac{\dfrac{\left(2R+288\right)}{3}}{\dfrac{\left(2R+48\right)}{3}+980}.100=12,9\\ \Leftrightarrow R=56\left(Fe\right)\\ \rightarrow Oxit:Fe_2O_3\)
Câu 7:
\(a.n_{NaOH}=\dfrac{60.10\%}{40}=0,15\left(mol\right)\)
Đặt \(C\%_{HCl}=a\left(\%\right)\Rightarrow n_{HCl}=\dfrac{40a}{100.36,5}=\dfrac{4a}{365}\left(mol\right)\)
\(C\%_{NaCl}=5,85\%\Leftrightarrow\dfrac{m_{NaCl}}{60+40}.100=5,85\Leftrightarrow m_{NaCl}=5,85\left(g\right)\Leftrightarrow n_{NaCl}=0,1\left(mol\right)\)
\(PTHH:NaOH+HCl\rightarrow NaCl+H_2O\)
(mol) 0,1 0,1 0,1
Lúc này ta có: \(n_{HCl}=\dfrac{4a}{365}=0,1\Leftrightarrow a=9,125\left(\%\right)\)
Câu b làm tương tự!!!

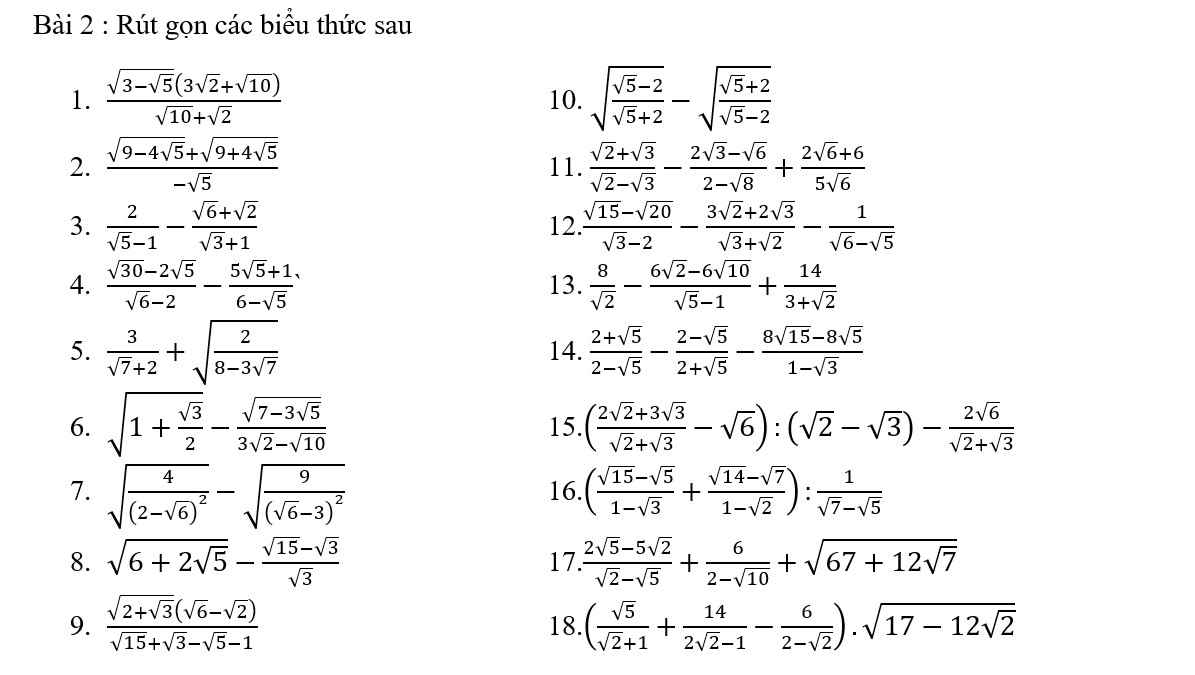
8: Ta có: \(\sqrt{6+2\sqrt{5}}-\dfrac{\sqrt{15}-\sqrt{3}}{\sqrt{3}}\)
\(=\sqrt{5}+1-\sqrt{5}+1\)
=2

Bài 2:
\(a,\Rightarrow x=\left(3,25\right):\left(0,15\right)\cdot\left(-1,2\right)=-26\\ b,\Rightarrow\left|3-2x\right|=4\Rightarrow\left[{}\begin{matrix}3-2x=4\\2x-3=4\end{matrix}\right.\Rightarrow\left[{}\begin{matrix}x=-\dfrac{1}{2}\\x=\dfrac{7}{2}\end{matrix}\right.\)
\(c,\) Áp dụng t/c dtsbn:
\(\dfrac{x}{3}=\dfrac{y}{5}=\dfrac{z}{4}=\dfrac{x+3y-2z}{3+15-8}=\dfrac{20}{10}=2\\ \Rightarrow\left\{{}\begin{matrix}x=6\\y=10\\z=8\end{matrix}\right.\)
\(d,\dfrac{x}{y}=\dfrac{5}{2}\Rightarrow\dfrac{x}{5}=\dfrac{y}{2};\dfrac{y}{z}=\dfrac{1}{3}\Rightarrow\dfrac{y}{1}=\dfrac{z}{3}\Rightarrow\dfrac{y}{2}=\dfrac{z}{6}\\ \Rightarrow\dfrac{x}{5}=\dfrac{y}{2}=\dfrac{z}{6}\)
Đặt \(\dfrac{x}{5}=\dfrac{y}{2}=\dfrac{z}{6}=k\Rightarrow x=5k;y=2k;z=6k\)
\(x^2-y^2+2z^2=372\\ \Rightarrow25k^2-4k^2+72k^2=372\\ \Rightarrow93k^2=372\Rightarrow k^2=4\\ \Rightarrow\left[{}\begin{matrix}k=2\\k=-2\end{matrix}\right.\Rightarrow\left[{}\begin{matrix}x=10;y=4;z=12\\x=-10;y=-4;z=-12\end{matrix}\right.\)










ddaay laf phaanf meemf team