Tính phần trăm khối lượng của các nguyên tố trong hợp chất : NaNO3, K2CO3, Al(OH)3
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Tính phần trăm khối lượng của các nguyên tố trong hợp chất: NaNO3; K2CO3 , Al(OH)3, SO2, SO3, Fe2O3.

\(NaNO_3\\ \%m_{Na}=\dfrac{23}{23+14+3.16}.100\approx27,059\%\\ \%m_N=\dfrac{14}{23+14+3.16}.100\approx16,471\%\\ \%m_O=\dfrac{3.16}{23+14+3.16}.100\approx56,471\%\)
Em tương tự làm cho các chất còn lại!

\(PTK_{NaNO_3}=23+14+3.16=85\left(đvC\right)\)
\(\%m_{Na}=\dfrac{23}{85}.100=27,05\%\)
\(\%m_N=\dfrac{14}{85}.100=16,47\%\)
\(\%m_O=\dfrac{3.16}{85}=56,47\%\)
\(PTK_{K_2CO_3}=2.39+12+3.16=138\left(đvC\right)\)
\(\%m_K=\dfrac{78}{138}.100=56,52\%\)
\(\%m_C=\dfrac{12}{138}.100=8,69\%\)
\(\%m_O=\dfrac{3.16}{138}.100=34,78\%\)
các ý còn lại làm tương tự
bạn cho mik thêm chỗ này nhé, lúc đấy vội nên mik ghi thiếu
\(\%m_O=\dfrac{3.16}{85}.100=56,47\%\)

Câu 2:
\(CTHH:X_2O_5\\ M_{X_2O_5}=\dfrac{16}{100\%-43,67\%}=142\left(g\text{/}mol\right)\\ \Rightarrow M_X=\dfrac{142-16.5}{2}=31\left(g\text{/}mol\right)\left(P\right)\\ \Rightarrow CTHH:P_2O_5\)
Câu 3:
Trong 1 mol B: \(\left\{{}\begin{matrix}n_{Al}=\dfrac{342.15,79\%}{27}=2\left(mol\right)\\n_S=\dfrac{342.28,07\%}{32}=3\left(mol\right)\\n_O=\dfrac{342-2.27-3.32}{16}=12\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow CTHH_B:Al_2\left(SO_4\right)_3\)
Câu 4:
\(M_X=8,5.2=17\left(g\text{/}mol\right)\)
Trong 1 mol X: \(\left\{{}\begin{matrix}n_N=\dfrac{17.82,35\%}{14}=1\left(mol\right)\\n_H=\dfrac{17.17,65\%}{1}=3\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow CTHH_X:NH_3\)
C1:
\(NaNO3:\)
\(MNaNO3=23+62=\dfrac{85g}{mol}\)
\(\%Na=\dfrac{23.100}{85}=27\%\)
\(\%N=\dfrac{14.100}{85}=16\%\)
\(\%O=\dfrac{16.3.100}{85}=56\%\)
\(K2CO3\)
\(MK2CO3=39.2+60=\dfrac{138g}{mol}\)
\(\%K=\dfrac{39.2.100}{138}=57\%\)
\(\%C=\dfrac{12.100}{138}=9\%\)
\(\%O=\dfrac{16.3.100}{138}=35\%\)
\(Al\left(OH\right)3:\)
\(MAl\left(OH\right)3=27+17.3=\dfrac{78g}{mol}\)
\(\%Al=\dfrac{27.100}{78}=35\%\)
\(\%O=\dfrac{16.3.100}{78}=62\%\)
\(\%H=\dfrac{1.3.100}{78}=4\%\)
\(SO2:\)
\(MSO2=32+16.2=\dfrac{64g}{mol}\)
\(\%S=\dfrac{32.100}{64}=50\%\)
\(\%O=\dfrac{16.2.100}{64}=50\%\)
\(SO3:\)
\(MSO3=32+16.3=\dfrac{80g}{mol}\)
\(\%S=\dfrac{32.100}{80}=40\%\)
\(\%O=\dfrac{16.3.100}{80}=60\%\)
\(Fe2O3:\)
\(MFe2O3=56.2+16.3=\dfrac{160g}{mol}\)
\(\%Fe=\dfrac{56.2.100}{160}=70\%\)
\(\%O=\dfrac{16.3.100}{160}=30\%\)
C5:
a,MX=2,207.29=64đvC
b, gọi cthh của hợp chất này là SxOy
Ta có: 32x:16y=50:50
=>x:y=\(\dfrac{50}{32}:\dfrac{50}{16}\)
= 1,5625:3,125
= 1 : 2
Vậy CTHH của hợp chất này là SO2
C2,3,4 lm r nên t bổ sung thim:>

Câu 1 :
\(M_{K_2CO_3}=39.2+12+16.3=138\left(dvC\right)\)
\(\%K=\dfrac{39.2}{138}.100\%=56,52\%\)
\(\%C=\dfrac{12}{138}.100\%=8,69\%\)
\(\%O=100\%-56,52\%-8,69\%=34,79\%\)
Còn lại cậu làm tương tự nhá

\(M_{MgSO_4}=24+32+16.4=120\\ \%Mg=\dfrac{24}{120}.100=20\%\\ \%S=\dfrac{32}{120}.100=26,67\%\\ \%O=\dfrac{16.4}{120}.100=53,33\%\\ M_{Al\left(NO_3\right)_3}=27+62.3=213\\ \%Al=\dfrac{27}{213}.100=12,68\%\\ \%N=\dfrac{14.3}{213}.100=19,72\%\\ \%O=\dfrac{16.9}{213}.100=67,6\%\)

\(MgSO_4=120\)
\(\%Mg=\dfrac{24}{120}.100\%=20\%\)
\(\%S=\dfrac{32}{120}.100\%\text{≈}26,67\%\)
\(\%O=100-\left(20+26,67\right)\text{≈}53,33\%\)
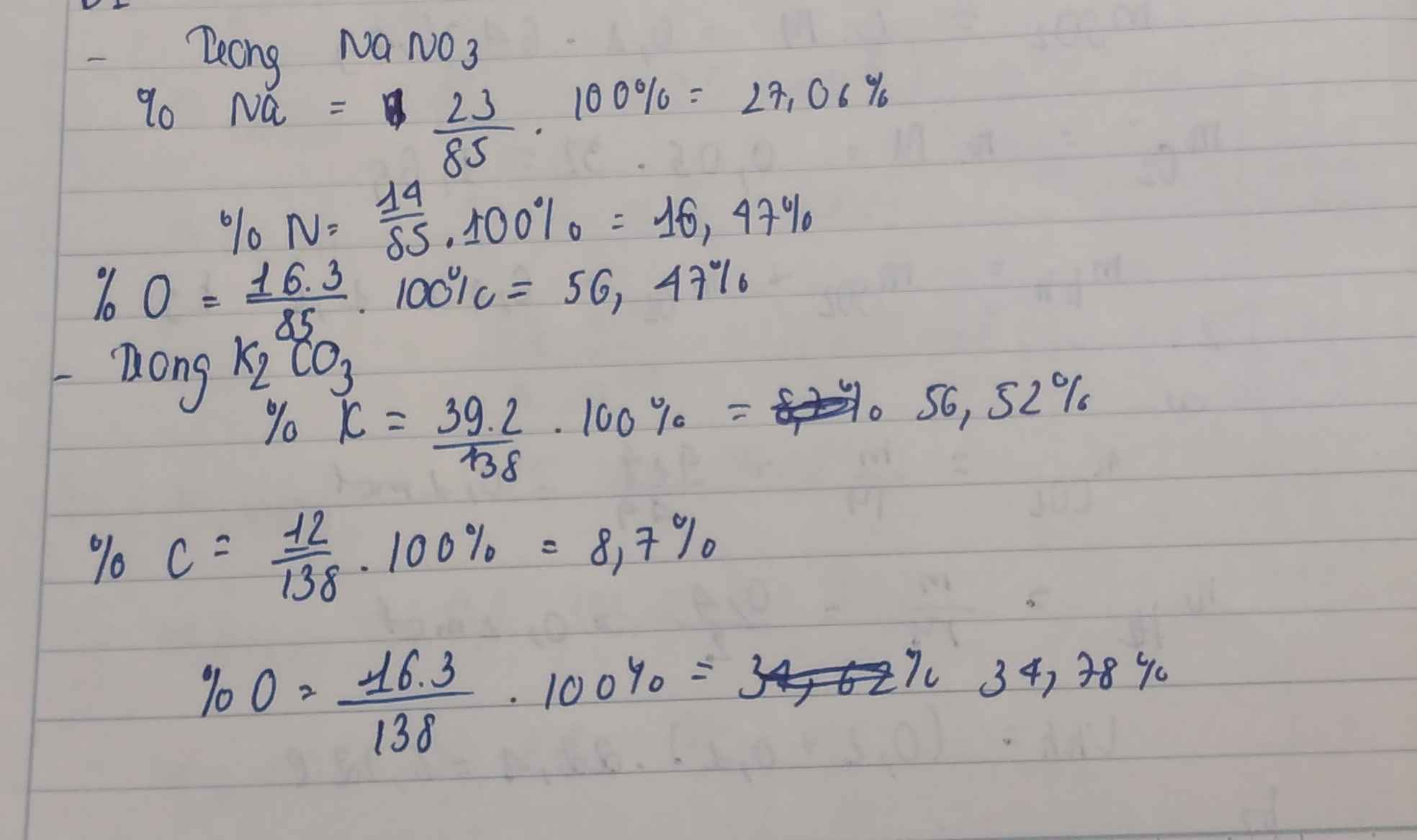
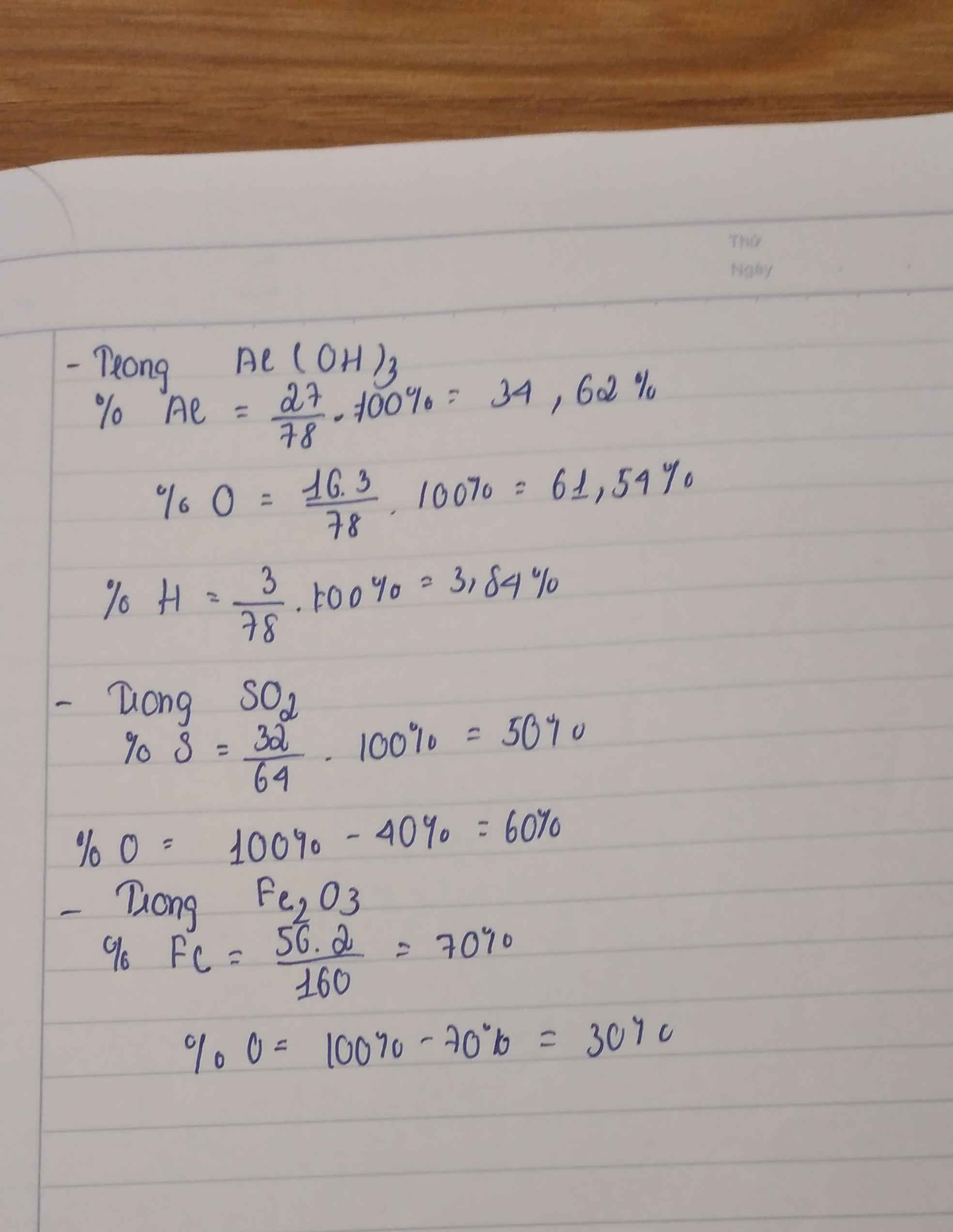
\(M_{NaNO_3}=23+14+16\times3=85\left(g\right)\)
\(\%Na=\dfrac{23}{85}\times100\%=27,06\%\)
\(\%N=\dfrac{14}{85}\times100\%=16,47\%\)
\(\%O=100\%-27,06\%-16,47\%=56,47\%\)
\(M_{K_2CO_3}=39\times2+12+16\times3=138\left(g\right)\)
\(\%K=\dfrac{39\times2}{138}\times100\%=56,52\%\)
\(\%C=\dfrac{12}{138}\times100\%=8,7\%\)
\(\%O=100\%-8,7\%-56,52\%=34,78\%\)
\(M_{Al\left(OH\right)_3}=27+3\times17=78\left(g\right)\)
\(\%Al=\dfrac{27}{78}\times100\%=34,62\%\)
\(\%O=\dfrac{16\times3}{78}\times100\%=61,54\%\)
\(\%H=100\%-34,62\%-61,54\%=3,84\%\)
* \(M_{Na\left(NO_3\right)}=23+14+48=85\left(g/mol\right)\)
\(\%Na=\dfrac{23}{85}.100\%=27,05\%\)
\(\%N=\dfrac{14}{85}.100\%=16,47\%\)
\(\Rightarrow\%O=100\%-\left(27,05+16,47\right)\%=56,48\%\)
* \(M_{K_2CO_3}=78+12+48=138\left(g/mol\right)\)
\(\%K=\dfrac{78}{138}.100\%=56,52\%\)
\(\%C=\dfrac{12}{138}.100\%=8,69\%\)
\(\Rightarrow\%O=100\%-\left(56,52+8,69\right)\%=34,79\%\)
* \(M_{Al\left(OH\right)_3}=27+48+3=78\left(g/mol\right)\)
\(\%Al=\dfrac{27}{78}.100\%=34,61\%\)
\(\%O=\dfrac{48}{78}.100\%=61,53\%\)
\(\Rightarrow\%H=100\%-\left(34,61+61,53\right)\%=3,86\%\)