Giúp mình với ạ mình đang cần gấp ạ cảm ơn
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.




1. How many kilos of potatoes would you like?
2. I went fishing but I didn't catch any fish.
3. Cook the chicken over low heat for 15 minutes before you serve it.
4. Can you tell me how to cook broken rice?
5. There are three cartons of milk in the fridge.
6. He has an egg but he hasn't got any bread.
7. Pho is one of the most popular dishes in Vietnam.



V Transformation
1 In spite being quite poor, the villagers live a happy and healthy way
2 Despite studying very hard, he still didn't pass the exam
3 Although Sylvia had no interest in folklore, she still enjoyed the story
4 Despite having much experience in machinery, he didn't succeed in repairing this machine
5 Though it was dark, they continued to work
6 In spite of having health problems, he is always smiling
7 Despite the difficult exam, Kieu Anh got good marks
8 THe man about whom I told you works in the hospital
9 Do you know the girl to whom Tom is talking?
10 The tree which stankds near the gate of my house has lovely flowers
11 The book which I was reading yesterday was a lovely story
12 Do you know the new student whose name I can't remember?
13 I will never forget the day when I first met her
14 The country where I was born is beautiful
15 Because of the polluted water, it was unsafe to drink
16 Because he work hard and methodically, John succeeded in his exam
17 I suggest getting together and talking about our presentation before we do it in class
18 When did you built this stilt house?
19 If you don't hurry up, you will be late for school
20 You must turn off the TV before 11p.m


PTHH: Al2O3+6HCl➝2AlCl3+3H2O(1)
a)nAl2O3=\(\dfrac{10,2}{102}\)=0,1(mol)
mHCl=\(\dfrac{5\%.219}{100\%}\)=10,95(g)
⇒nHCl=\(\dfrac{10,95}{36,5}\)=0,3(mol)
Xét tỉ lệ Al2O3:\(\dfrac{0,1}{1}\)=0,1
Xét tỉ lệ HCl:\(\dfrac{0,3}{6}\)=0,05
⇒HCl pứng hết,Al2O3 còn dư
Theo PTHH(1) ta có nAl2O3 pứng=\(\dfrac{nHCl}{6}\)=\(\dfrac{0,3}{6}\)=0,05(mol)
⇒nAl2O3 dư=nAl2O3ban đầu-nAl2O3 pứng=0,1-0,05=0,05(mol)
⇒mAl2O3 dư=0,05.102=5,1(g)
b) C%HCl=\(\dfrac{0,3.36,5}{219+10,2}\).100%=4,8%
nAlCl3=0,1(mol)
⇒C%AlCl3=\(\dfrac{0,1.136,5}{10,2+219}\).100%=6%




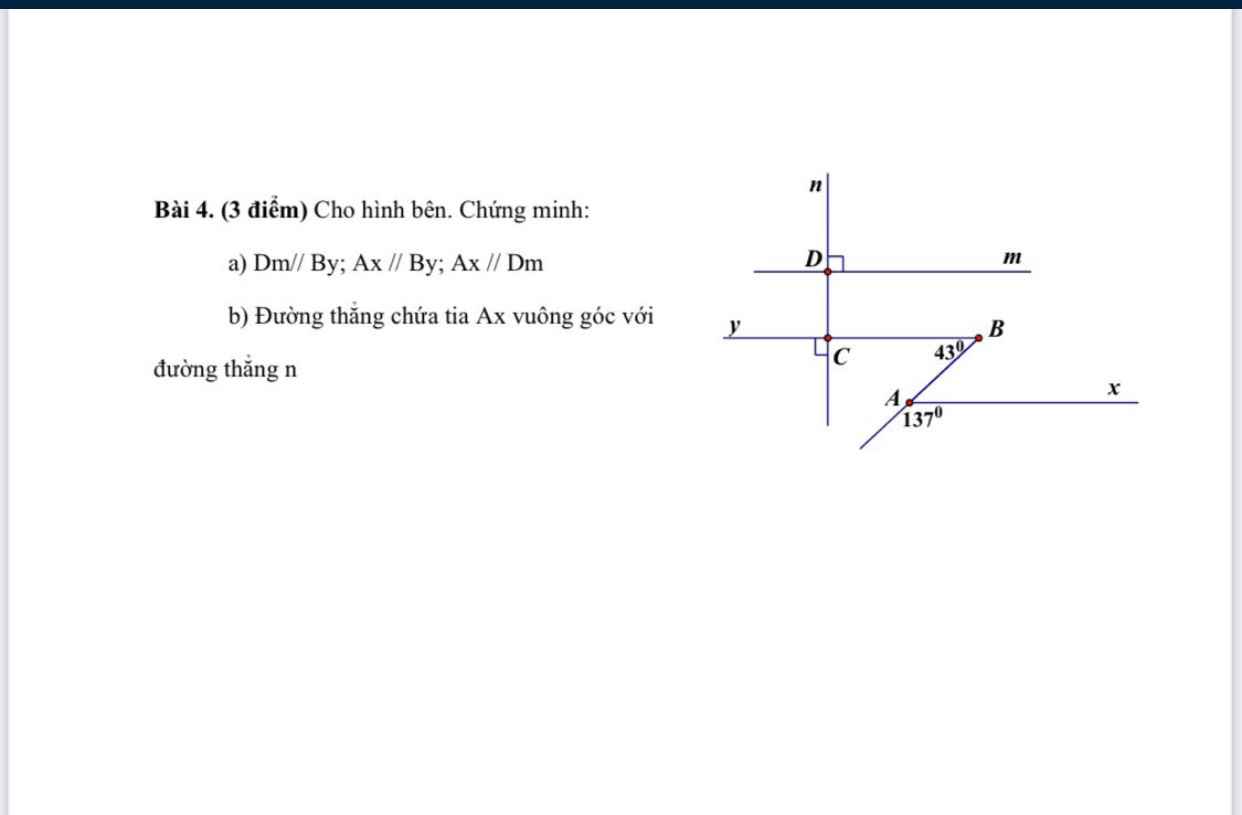
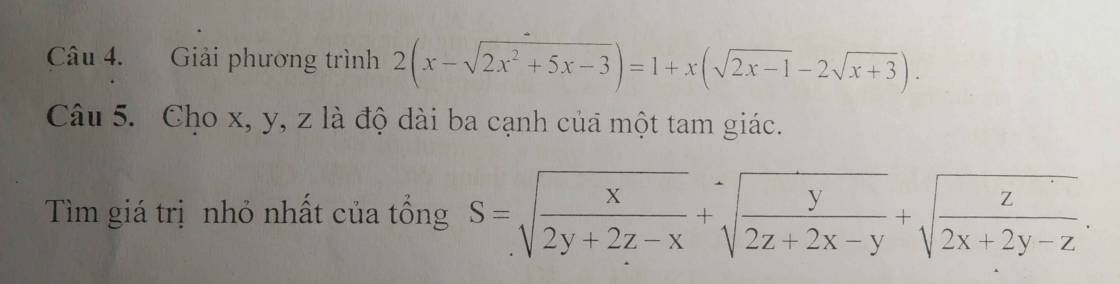

 giúp mình với ạ, mình đang cần gấp, cảm ơn trc ạ
giúp mình với ạ, mình đang cần gấp, cảm ơn trc ạ Giúp mình với ạ mình đang cần gấp lắm :'(( mình cảm ơn ạ
Giúp mình với ạ mình đang cần gấp lắm :'(( mình cảm ơn ạ

1) \(\left(\dfrac{-13}{17}-\dfrac{31}{52}\right)-\left(\dfrac{73}{52}-\dfrac{13}{17}+\dfrac{5}{6}\right)-\dfrac{3}{4}\)
\(=\dfrac{-13}{17}-\dfrac{31}{52}-\dfrac{73}{52}+\dfrac{13}{17}-\dfrac{5}{6}-\dfrac{3}{4}\)
\(=\left(\dfrac{-13}{17}+\dfrac{13}{17}\right)-\left(\dfrac{31}{52}+\dfrac{73}{52}\right)-\left(\dfrac{5}{6}+\dfrac{3}{4}\right)\)
\(=0-2-\dfrac{19}{12}\)
\(=-2-\dfrac{19}{12}\)
\(=\dfrac{-43}{12}\)
2) \(\dfrac{1}{7}.\dfrac{1}{3}+\dfrac{1}{7}.\dfrac{1}{2}-\dfrac{1}{7}\)
\(=\dfrac{1}{7}\left(\dfrac{1}{3}+\dfrac{1}{2}-1\right)\)
\(=\dfrac{1}{7}.-\dfrac{1}{6}\)
\(=-\dfrac{1}{42}\)