GIÚP MÌNH CÂU 1 --> 8 VỚI!!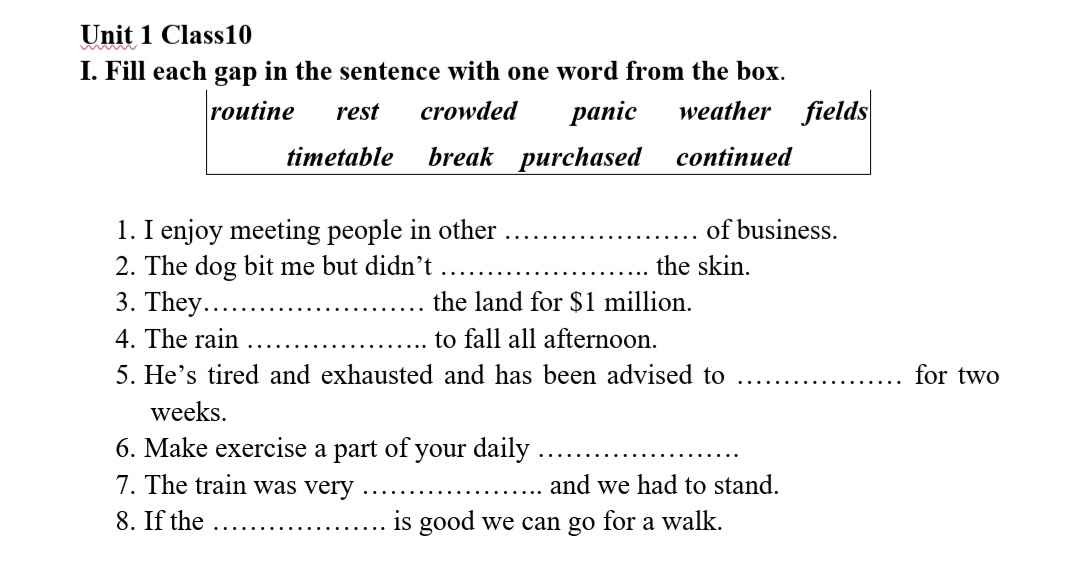
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


Đặt \(A=\frac{1}{2}+\frac{1}{4}+\frac{1}{8}+...+\frac{1}{1024}\)
\(2A=1+\frac{1}{2}+\frac{1}{4}+\frac{1}{8}+...+\frac{1}{1024}\)
\(2A-A=\left(1+\frac{1}{2}+\frac{1}{4}+\frac{1}{8}+...+\frac{1}{1024}\right)-\left(\frac{1}{2}+\frac{1}{4}+\frac{1}{8}+...+\frac{1}{1024}\right)\)
\(\Rightarrow A=1-\frac{1}{1024}=\frac{1023}{1024}\)

\(n_{Zn}=\dfrac{3,25}{65}=0,05\left(mol\right)\)
pthh : \(Zn+2HCl->ZnCl_2+H_2\)
0,05 0,05
\(n_{CuO}=\dfrac{6}{80}=0,075\left(mol\right)\)
pthh : \(H_2+CuO-t^o->Cu+H_2O\)
LTL :
\(\dfrac{0,05}{1}< \dfrac{0,075}{1}\)
=> CuO dư
theo pthh : nCu = nH2 =0,05 (mol)
=> \(m_{Cu}=0,05.64=3,2\left(g\right)\)
theo pthh : \(n_{CuO\left(p\text{ư}\right)}=n_{H_2}=0,05\left(mol\right)\)
=> \(n_{CuO\left(d\right)}=0,075-0,05=0,025\left(mol\right)\)
=>\(m_{CuO}=0,025.80=2\left(g\right)\)
\(n_{Al}=\dfrac{2,7}{27}=0,1\left(mol\right)\)
PTHH: 2Al + 3H2SO4 ---> Al2(SO4)3 + 3H2
0,1 0,15 0,05 0,15
PbO + H2 --to--> Pb + H2O
0,15 0,15
\(\rightarrow\left\{{}\begin{matrix}a=0,15.98=14,7\left(g\right)\\V=0,15.22,4=3,36\left(l\right)\\m_{PbO}=0,15.233=34,95\left(g\right)\end{matrix}\right.\)

Câu 8 :
a) \(n_{Cu}=\dfrac{6,4}{64}=0,1\left(mol\right)\)
\(n_{Al}=\dfrac{54}{27}=2\left(mol\right)\)
b) \(V_{CO_2}=0,15.22,4=3,36\left(l\right)\)
\(V_{N_2}=0,3.22,4=6,72\left(l\right)\)
c) \(n_{hh}=n_{CO_2}+n_{H_2}=\dfrac{0,22}{44}+\dfrac{0,02}{2}=0,015\left(mol\right)\)
\(V_{hh}=0,015.22,4=0,336\left(l\right)\)
Câu 9
a) \(m_N=0,3.14=4.2\left(g\right)\)
\(m_{Cl}=0,4.35,5=14,2\left(g\right)\)
\(m_O=5.16=80\left(g\right)\)
b) \(m_{N_2}=0,2.28=5,6\left(h\right)\)
\(m_{Cl_2}=0,3.71=21,3\left(g\right)\)
\(m_{O_2}=4.32=128\left(g\right)\)
c) \(m_{Fe}=0,12.56=6,72\left(g\right)\)
\(m_{Cu}=3,15.64=201,6\left(g\right)\)
\(m_{H_2SO_4}=0,85.98=83,3\left(g\right)\)
\(m_{CuSO_4}=0,52.160=83,2\left(g\right)\)

 Giúp mình câu 8 với câu 10 ik
Giúp mình câu 8 với câu 10 ik giúp mình với mình chỉ cần 2 câu 8 với câu 9 thôi nha
giúp mình với mình chỉ cần 2 câu 8 với câu 9 thôi nha 



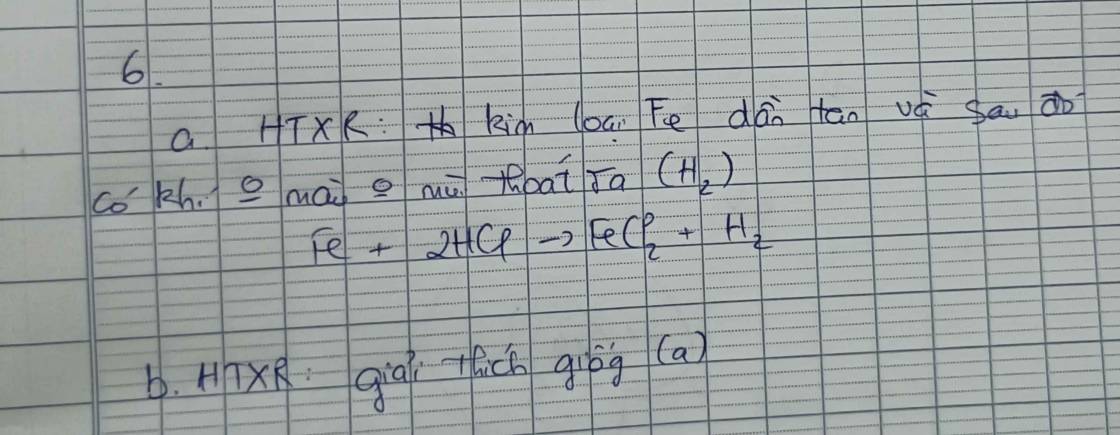


1 fields
2 break
3 purchased
4 continued
5 rest
6 routine
7 crowded
8 weather