Trộn 200g SO3 vào 1 lít dd H2SO4 17% có KLR D= 1,12 g/ml. Tính nồng độ % của dd H2SO4 thu được
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

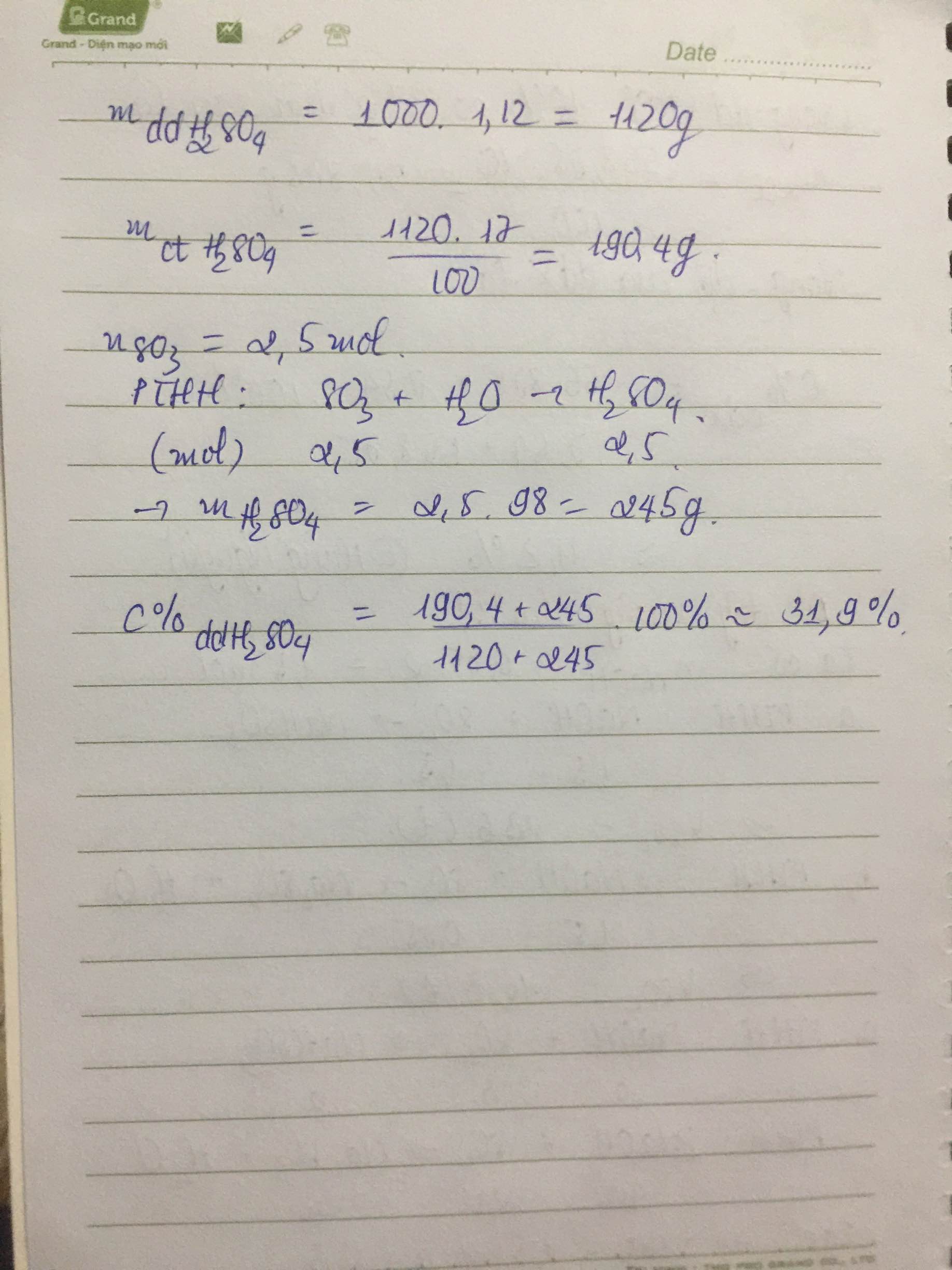

\(n_{SO_3}=\dfrac{200}{80}=2,5\left(mol\right)\)
mdd H2SO4 17% = 1000.1,12 = 1120 (g)
=> \(m_{H_2SO_4}=\dfrac{1120.17}{100}=190,4\left(g\right)\)
PTHH: SO3 + H2O --> H2SO4
2,5------------>2,5
=> mH2SO4(sau pư) = 2,5.98 + 190,4 = 435,4 (g)
mdd sau pư = 200 + 1120 = 1320 (g)
\(C\%_{dd.H_2SO_4.sau.pư}=\dfrac{435,4}{1320}.100\%=32,985\%\)

SO3 + H2O → H2SO4
\(m_{ddH_2SO_4.17\%}=1000\times1,12=1120\left(g\right)\)
\(\Rightarrow m_{H_2SO_4.17\%}=1120\times17\%=190,4\left(g\right)\)
\(n_{SO_3}=\dfrac{200}{80}=2,5\left(mol\right)\)
Theo PT: \(n_{H_2SO_4}tt=n_{SO_3}=2,5\left(mol\right)\)
\(\Rightarrow m_{H_2SO_4}tt=2,5\times98=245\left(g\right)\)
\(\Rightarrow m_{H_2SO_4}mới=m_{H_2SO_4}tt+m_{H_2SO_4.17\%}=245+190,4=435,4\left(g\right)\)
\(m_{dd}mới=m_{SO_3}+m_{ddH_2SO_4.17\%}=200+1120=1320\left(g\right)\)
\(\Rightarrow C\%_{dd}mới=\dfrac{m_{H_2SO_4}mới}{m_{dd}mới}=\dfrac{435,4}{1320}\times100\%=32,98\%\)
PTHH: \(SO_3+H_2O\rightarrow H_2SO_4\\ 2,5mol:2,5mol\rightarrow2,5mol\)
\(n_{SO_3}=\dfrac{200}{80}=2,5\left(mol\right)\)
\(m_{H_2SO_4}=2,5.98=245\left(g\right)\)
\(m_{ddH_2SO_417\%}=1000.1,12=1120\left(g\right)\)
\(m_{H_2SO_4trongdd}=17\%.1120=190,4\left(g\right)\)
\(C\%dd=\dfrac{245+190,4}{1120+200}.100\%=32,98\%\)

\(n_{NaOH}=0,2.1=0,2\left(mol\right)\\ n_{H_2SO_4}=0,3.1,5=0,45\left(mol\right)\)
\(2NaOH+H_2SO_4\rightarrow Na_2SO_4+2H_2O\)
0,2------->0,1--------->0,1
Xét \(\dfrac{0,2}{2}< \dfrac{0,45}{1}\Rightarrow\) \(H_2SO_4\)dư
Trong dung dịch D có:
\(\left\{{}\begin{matrix}n_{H_2SO_4}=0,45-0,1=0,35\left(mol\right)\\n_{Na_2SO_4}=0,1\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}CM_{H_2SO_4}=\dfrac{0,35}{0,5}=0,7M\\CM_{Na_2SO_4}=\dfrac{0,1}{0,5}=0,2M\end{matrix}\right.\)
b
\(Ca\left(OH\right)_2+H_2SO_4\rightarrow CaSO_4+2H_2O\)
0,35<---------0,35
\(V_{Ca\left(OH\right)_2}=\dfrac{0,35.74}{1,2}=\dfrac{259}{12}\approx21,58\left(ml\right)\\ \Rightarrow V_{dd.Ca\left(OH\right)_2}=\dfrac{\dfrac{259}{12}.100\%}{10\%}=\dfrac{1295}{6}\approx215,83\left(ml\right)\)

nK = 39 / 39=1 (mol)
Pt: 2K + 2H2O --> 2KOH + H2
1 mol--------------------------> 0,5 mol
mH2 = 0,5 . 2 = 1 (g)
mdd = mK + mnước - mH2 = 39 + 362 - 1 = 400 (g)
C% dd KOH = 39/400.100%=9,75%
https://hoc24.vn/hoi-dap/tim-kiem?id=562460&q=t%C3%ADnh%20n%E1%BB%93ng%20%C4%91%E1%BB%99%20ph%E1%BA%A7n%20tr%C4%83m%20c%E1%BB%A7a%20dung%20d%E1%BB%8Bch%20t%E1%BB%8Da%20th%C3%A0nh%20khi%20h%C3%B2a%20tan%20%3A%20%201%2F%2039g%20Kali%20v%C3%A0o%20362g%20n%C6%B0%E1%BB%9Bc%20%202%2F200g%20So3%20v%C3%A0o%201%20l%C3%ADt%20dung%20d%E1%BB%8Bch%20H2SO4%2017%25%20%28D%20%3D%201%2C12%20G%2FML%29