Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


Mark the letter A, B, C or D on your answer sheet to indicate the word(s) CLOSEST in meaning
18. Every month, the volunteer group go to remote and mountainous areas to help those in need.
A. Empty B. faraway C. crowded D. poor
19. Women will be exhausted if they have to cover both jobs at work and at home.
A. Very relaxed B. very pleased C. very tired D. very happy
Mark the letter A, B, C or D on your answer sheet to indicate the word(s) OPPOSITE in meaning
20. Name some famous football players in the world.
A. infamous B. unknown C. impossible D. irregular
21. In my view, husbands should contribute to the household duties in order to reduce burden on their wives.
A. Minimize B. lower C. decrease D. increase
Mark the letter A, B, C or D on your answer sheet to indicate the most suitable response
22. The waiter: “coffee or lemon juice”_- Mr Hung : “ ……………”________________
A. I’ll have lemon juice, please B. coffee is not expensive
C. What did I ? D, No, thank you
23. “What did Mr Nam talk about?”_______________
A. He has a good job B. His parents are now in London
C. His family. D. He has a big family
Mark the letter A,B,C or D to indicate the word that differs from the other three in the position of primary stress in each of the following questions.
3. A. driverless B. wonderland C. favourite D. expensive
4. A. festival B. disaster C. pavement D. station



ta có nAl(OH)3(1)= 6,24/78= 0,08 (mol); nNaOH(1)= 0,24*1= 0,24 (mol);
AlCl3 + 3NaOH --> Al(OH)3 + 3NaCl; (1)
0,08------0,24---------0,08
ta có NaOH hết.
Al(OH)3 + NaOH---> NaAlO2 + 2H2O; (2)
0,06----------0,06 (mol)
AlCl3 + 3NaOH --> Al(OH)3 + 3NaCl; (3)
0,013-----0,04 (mol)
ta có nAl(OH)3 sau pư= 4,68/78= 0,06 (mol);
=> nNaOH(2)= 0,06 (mol)
ta có nNaOH thêm vào= 0,1*1=0,1 (mol)
=> nNaOH(3)=0,1-0,06=0,04 (mol);
=> nAlCl3( trong X)=0,08+ 0,013=0,093(mol);
CM (X)= 0,093/0,1= 0,93 (M

a.
CnH2n+H2Ni,to⟶CnH2n+2CmH2m−2+2H2Ni,to⟶CmH2m+2{CnH2n:xCmH2m−2:y→{x+y=100x+2y=160→{x=40y=60→%mol=%V{40%60%CnH2n+H2⟶Ni,toCnH2n+2CmH2m−2+2H2⟶Ni,toCmH2m+2{CnH2n:xCmH2m−2:y→{x+y=100x+2y=160→{x=40y=60→%mol=%V{40%60%
b.
Khi cho NaOH dư vào thu thêm được kết tủa nên dung dịch có muối Ca(HCO3)2.
CO2 + Ca(OH)2 → CaCO3↓ + H2O
0,5 ← 0,15
2CO2 + Ca(OH)2 → Ca(HCO3)2
Ca(HCO3)2 + 2NaOH → CaCO3↓ + Na2CO3 + 2H2O
0,1 ← 0,1
→ nCO2 = 0,1.2 + 0,5 = 0,7
Mặt khác: mdd giảm = mCaCO3 – mCO2 + mH2O
→ 9,12 = 50 – (44.0,7 + 18.nH2O) → nH2O = 0,56
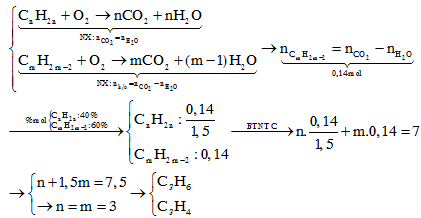
\(\text{Theo đề ra, ta có:}\)\(\text{Một hợp chất chứa 40%Cu}\)\(,\)\(\text{20%S còn lại là O}\)
\(\rightarrow\%O=100\%-40\%-20\%=40\%\)
\(Cu:S:O=\frac{40}{64}=\frac{20}{32}=\frac{40}{16}=1:1:4\)
\(\rightarrow CTHH\)\(\text{của hợp chất là CuSO4}\)
Đề không cho %S nhé bạn