
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


Gọi $n_{H_2} = a(mol) ; n_{CO} = b(mol) ; n_{CO_2} = c(mol)$
$\Rightarrow 2a + 28b + 44c = 3,72(1)$
Mặt khác :
$n_{hh} = \dfrac{13,44}{22,4} = 0,6(mol)$
$H_2 + CuO \xrightarrow{t^o} Cu + H_2O$
$CO + CuO \xrightarrow{t^o} Cu + CO_2$
$n_{CuO} = n_{H_2} + n_{CO}$
Ta có : $\dfrac{a + b + c}{a + b} = \dfrac{0,6}{0,5}$
$\Rightarrow -0,1a -0,1b + 0,5c = 0(2)$
$C + H_2O \xrightarrow{t^o} CO + H_2$
$C + 2H_2O \xrightarrow{t^o} CO_2 + 2H_2$
Theo PTHH : $n_{H_2} = n_{CO} + 2n_{CO_2}$
$\Rightarrow a = b + 2c(3)$
Từ (1)(2)(3) suy ra : a = 0,14 ; b = 0,06 ; c = 0,04
$n_{H_2O} = n_{H_2} = 0,14(mol)$
$m = 0,14.18 = 2,52(gam)$
\(n_X=\dfrac{13,44}{22,4}=0,6\left(mol\right)\)
Đặt \(\left\{{}\begin{matrix}n_{H_2}=x\\m_{CO}=y\\n_{CO_2}=z\end{matrix}\right.\) ( mol )
\(\Rightarrow n_X=x+y+z=0,6\left(mol\right)\left(1\right)\)
\(C+H_2O\rightarrow\left(t^o\right)CO+H_2\)
\(C+2H_2O\rightarrow\left(t^o\right)CO_2+2H_2\)
\(Cu+\left\{{}\begin{matrix}CO\\H_2\end{matrix}\right.\rightarrow\left(t^o\right)CuO+\left\{{}\begin{matrix}CO_2\\H_2\end{matrix}\right.\)
\(n_{CuO}=x+y=\dfrac{40}{80}=0,5\left(mol\right)\left(2\right)\)
\(n_{H_2}=n_{CO}+2n_{CO_2}\)
\(\Rightarrow x=y+2z\left(3\right)\)
\(\left(1\right);\left(2\right);\left(3\right)\rightarrow\left\{{}\begin{matrix}x=0,35\\y=0,15\\z=0,1\end{matrix}\right.\)
\(m_X=m_{H_2}+m_{CO}+m_{CO_2}\)
\(=0,35.2+0,15.28+0,1.44=9,3\left(g\right)\)
Bảo toàn H: \(n_{H_2O}=n_{H_2}=0,35\left(mol\right)\) \(\Rightarrow m_{H_2O}=0,35.18=6,3\left(g\right)\)
Ta có: 9,3 gam X `->` 6,3 gam H2O
3,72 gam X `->` 2,52 gam H2O
`=>` \(m=2,52\left(g\right)\)

Gọi kim loại cần tìm là R
Đặt \(n_{R\left(NO_3\right)_2}=n_{RCl_2}=a\left(mol\right)\)
`=>` \(\left\{{}\begin{matrix}m_{R\left(NO_3\right)_2}=a.\left(M_R+124\right)\left(g\right)\\m_{RCl_2}=a.\left(M_R+71\right)\left(g\right)\end{matrix}\right.\)
`=>` \(m_{R\left(NO_3\right)_2}>m_{RCl_2}\Rightarrow m_{R\left(NO_3\right)_2}=3,33+1,59=4,92\left(g\right)\)
`=>` \(\dfrac{m_{R\left(NO_3\right)_2}}{m_{RCl_2}}=\dfrac{a.\left(M_R+124\right)}{a.\left(M_R+71\right)}=\dfrac{4,92}{3,33}\)
`=>` \(\dfrac{M_R+124}{M_R+71}=\dfrac{4,92}{3,33}\)
`=>` \(M_R=40\left(g/mol\right)\)
`=> R: Ca`

Gọi kim loại cần tìm là A có hoá trị n
PTHH: \(2A+2nHCl\rightarrow2ACl_n+nH_2\)
Gọi \(n_{HCl}=1\left(mol\right)\Rightarrow n_{HCl\left(pư\right)}=\left(100\%-20\%\right).1=0,8\left(mol\right)\)
`=>` \(m_{ddHCl}=\dfrac{1.36,5}{3,65\%}=1000\left(g\right)\)
Theo PT: \(\left\{{}\begin{matrix}n_A=n_{ACl_n}=\dfrac{1}{n}n_{HCl}=\dfrac{0,8}{n}\left(mol\right)\\n_{H_2}=\dfrac{1}{2}n_{HCl}=0,4\left(mol\right)\end{matrix}\right.\)
`=>` \(m_{ddspư}=1000+\dfrac{0,8M_A}{n}-0,4.2=999,2+\dfrac{0,8M_A}{n}\left(g\right)\)
`=>` \(C\%_{ACl_n}=\dfrac{\dfrac{\dfrac{0,8}{n}.\left(M_A+35,5n\right)}{n}}{999,2+\dfrac{0,8M_A}{n}}.100\%=4,973\%\)
`=>` \(M_A=28n\left(g/mol\right)\)
`n = 2 => M_A = 56`
`=> A: Fe`

a) `n_{CaCO_3} = (50)/(100) = 0,5 (mol)`
PTHH: `CaCO_3 + 2HCl -> CaCl_2 + CO_2 + H_2O`
Theo PT: `n_{CO_2} = n_{CaCl_2} = n_{CaCO_3} = 0,5 (mol)`
`=> V = 0,5.22,4 = 11,2 (l)`
b) \(C_{M\left(CaCl_2\right)}=\dfrac{0,5}{0,5}=1M\)
c) PTHH: `2NaOH + CO_2 -> Na_2CO_3 + H_2O`
Theo PT: `n_{Na_2CO_3} = n_{CO_2} =0,5 (mol)`
`=> m_{Na_2CO_3} = 0,5.106 = 53 (g)`

a) \(n_{H_2SO_4}=\dfrac{200.4,9\%}{98}=0,1\left(mol\right)\)
PTHH: \(BaCl_2+H_2SO_4\rightarrow BaSO_4\downarrow+2HCl\) (1)
ban đầu 0,1 0,1
phản ứng 0,1 0,1
sau phản ứng 0 0 0,1 0,2
`=>` \(ddA\left\{{}\begin{matrix}FeCl_2:0,12\left(mol\right)\\AlCl_3:0,1\left(mol\right)\\HCl:0,2\left(mol\right)\end{matrix}\right.\)
Ta có: \(n_{NaOH}=\dfrac{37,6}{40}=0,94\left(mol\right)\)
Giả sử NaOH dư
PTHH:
\(HCl+NaOH\rightarrow NaCl+H_2O\) (2)
0,2---->0,2--------->0,2
\(FeCl_2+2NaOH\rightarrow Fe\left(OH\right)_2\downarrow+2NaCl\) (3)
0,12----->0,24---------->0,12--------->0,24
\(AlCl_3+3NaOH\rightarrow Al\left(OH\right)_3\downarrow+3NaCl\) (4)
0,1------>0,3---------->0,1----------->0,3
`=>` \(n_{NaOH\left(pư\right)}=0,2+0,24+0,3=0,74\left(mol\right)< 0,94\)
`=> NaOH` dư, giả ửu đúng
Tiếp tục xảy ra phản ứng: \(Al\left(OH\right)_3+NaOH\rightarrow NaAlO_2+2H_2O\) (5)
ban đầu 0,1 0,2
phản ứng 0,1--------->0,1
sau phản ứng 0 0,1 0,1
`=>` \(\left\{{}\begin{matrix}ddC\left\{{}\begin{matrix}NaCl:0,2+0,24+0,3=0,74\left(mol\right)\\NaOH:0,1\left(mol\right)\\NaAlO_2:0,1\left(mol\right)\end{matrix}\right.\\\downarrow B:BaSO_4,Fe\left(OH\right)_2\end{matrix}\right.\)
PTHH:
\(4Fe\left(OH\right)_2+O_2\xrightarrow[]{t^o}2Fe_2O_3+4H_2O\) (6)
0,12------------------->0,06
`=>` \(m_{c.rắn}=0,1.233+0,06.160=32,9\left(g\right)\)
b) \(m_{ddC}=200+0,12.127+0,1.133,5+0,1.208+37,6-0,1.233-0,12.90=252,89\left(g\right)\)
`=>` \(m_{H_2O\left(thêm\right)}=400-252,89=147,11\left(g\right)\)
`=>` \(\left\{{}\begin{matrix}C\%_{NaCl}=\dfrac{0,74.58,5}{400}.100\%=10,8225\%\\C\%_{NaOH}=\dfrac{0,1.40}{400}.100\%=1\%\\C\%_{NaAlO_2}=\dfrac{0,1.82}{400}.100\%=2,05\%\end{matrix}\right.\)

Động vật, cây cối, sông suối, ao hồ là những vật thể tự nhiên. Sách, vở, ti vi, xe máy, quạt điện là những vật thể nhân tạo

`a.` \(n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
0,1 0,1 ( mol )
\(Fe_xO_y+2yHCl\rightarrow xFeCl_{\dfrac{2y}{x}}+yH_2O\)
\(\Rightarrow m_{Fe}=0,1.56=5,6\left(g\right)\)
\(\Rightarrow m_{Fe_xO_y}=6,4-5,6=0,8\left(g\right)\)
Ta có: 3,2g hh + H2 `->` 0,1g H2O
\(\Rightarrow\) 6,4g hh + H2 `->` 0,2g H2O
\(n_{H_2O}=\dfrac{0,2}{18}=\dfrac{1}{90}\left(mol\right)\)
\(Fe_xO_y+yH_2\rightarrow\left(t^o\right)xFe+yH_2O\)
\(\Rightarrow n_{O\left(Fe_xO_y\right)}=n_{H_2O}=\dfrac{1}{90}\left(mol\right)\)
Ta có:\(m_{Fe_xO_y}=56x+16.\dfrac{1}{90}=0,8\)
\(\Leftrightarrow x=\dfrac{1}{90}\)
\(\Rightarrow x:y=\dfrac{1}{90}:\dfrac{1}{90}=1:1\)
\(\Rightarrow CTHH:FeO\)

Ta có: \(\left\{{}\begin{matrix}p+e+n=58\\p=e\\p+e-n=18\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}p=e=19\\n=20\end{matrix}\right.\)

Gọi \(n_{CuSO_4}=n_{CaCO_3}=a\left(mol\right)\)
`=> 160a + 100a = 52`
`=> a = 0,2 (mol) = n_{CuSO_4} + n_{CaCO_3}`
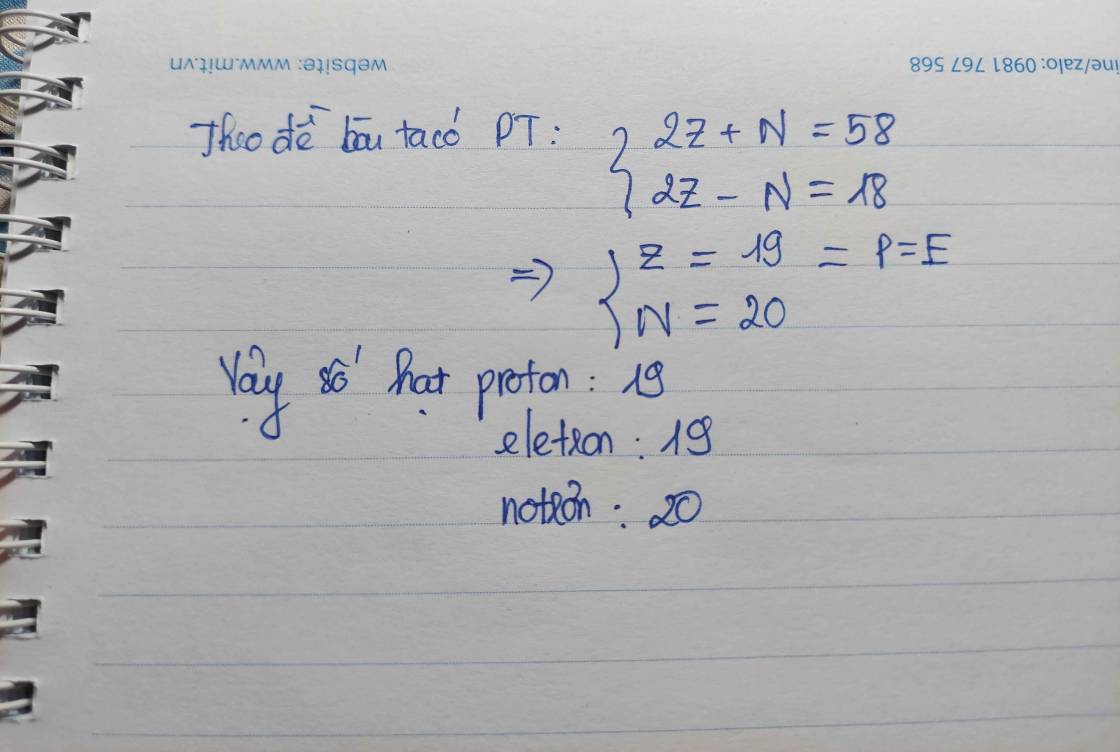
a) \(\overset{I}{X}_2O,\overset{II}{X}O,\overset{III}{X}_2O_3,\overset{IV}{X}O_2,\overset{V}{X}_2O_5,\overset{VI}{X}O_3,\overset{VII}{X}_2O_7\)
b) \(\overset{I}{X}H,\overset{II}{X}H_2,\overset{III}{X}H_3,\overset{IV}{X}H_4\)