Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(CaCO_3\\ \%m_{Ca}=\dfrac{40}{40+12+3.16}.100=40\%\\ \%m_C=\dfrac{12}{40+12+16.3}.100=12\%\\ \Rightarrow\%m_O=100\%-\left(40\%+12\%\right)=48\%\\ H_2SO_4\\ \%m_H=\dfrac{2.1}{2.1+32+4.16}.100\approx2,041\%\\ \%m_S=\dfrac{32}{2.1+32+4.16}.100\approx32,653\%\\ \%m_O=\dfrac{4.16}{2.1+32+4.16}.100\approx65,306\%\\ Fe_2O_3\\ \%m_{Fe}=\dfrac{56.2}{56.2+16.3}.100=70\%\\ \Rightarrow\%m_O=100\%-70\%=30\%\)
CaCO3
\(\%M_{\dfrac{Ca}{CaCO_3}}=\dfrac{40}{100}.100\%=40\%\)
\(\%M_{\dfrac{C}{CaCO_3}}=\dfrac{12}{100}.100\%=12\%\)
\(\%M_{\dfrac{O}{CaCO_3}}=100\%-\left(40\%+12\%\right)=48\%\)
H2SO4
\(\%M_{\dfrac{H_2}{H_2SO_4}}=\dfrac{2}{98}.100\%=2,04\%\)
\(\%M_{\dfrac{S}{H_2SO_4}}=\dfrac{32}{98}.100\%=32,65\%\)
\(\%M_{\dfrac{O}{H_2SO_4}}=100\%-\left(2,04\%+32,65\%\right)=65,31\%\)
Fe2O3
\(\%M_{\dfrac{Fe}{Fe_2O_3}}=\dfrac{112}{160}.100\%=70\%\)
\(\%M_{\dfrac{O}{Fe_2O_3}}=100\%-70\%=30\%\)

\(\%Fe=\dfrac{56}{56.2+16.3}.100\%=35\%\\ \%O=\dfrac{16}{56.2+16.3}.100\%=10\%\)

Khối lượng mol của \(Fe2O3\) là :
\(M_{Fe_2}_{O_3}=56,2+16,3=160\left(g.mol\right)\)
\(\%Fe=\dfrac{56.2}{100}.100\%=70\%\)
\(\%O=100\%-70\%=30\%\)

\(\%Fe=\dfrac{m_{Fe}}{M_{Fe_2O_3}}=\dfrac{112}{160}=70\%\\ \%O=100\%-\%Fe=100\%-70\%=30\%\)

CaCO3 có PTK =100g/mol
=> %Ca=\(\frac{40}{100}.100=40\%\)
%C=12:100.100=12%
%O=3.16:100.100=48%
H2SO4 có PTK =98
=> %H=2.1:98.100=2%
%S=32:98.100=32,7%
=> %O=100-2-32,7=65,3%
Fe2O3 có PTK =160
=> %Fe=56.2:160.100=70%
=> %O=100-70=30%
Mg(OH)2 có PTK: 58g/mol
=> %Mg=24:56.100=42,9%
%H=1.2:56.100=3,6%
=> %O=100-42,9-3,6=56,5%

a) \(\left\{{}\begin{matrix}\%Fe=\dfrac{56.2}{160}.100\%=70\%\\\%O=100\%-70\%=30\%\end{matrix}\right.\)
b) \(\left\{{}\begin{matrix}\%Al=\dfrac{27.2}{342}.100\%=15,79\%\\\%S=\dfrac{32.3}{342}.100\%=28,07\%\\\%O=\dfrac{16.12}{342}.100\%=56,14\%\end{matrix}\right.\)

a/
+ CO
- %mC = \(\frac{12}{12+16}.100\%=42,86\%\)
- %mO = 100% - 42,86% =57,14%
+CO2
- %mC = \(\frac{12}{12+16.2}.100\%=27,27\%\)
- %mO = 100% - 27,27% = 72,73%
b/
+Fe3O4
- %mFe = \(\frac{56.3}{56.3+16.4}.100\%=72,41\%\)
- %mO = 100% - 72,41% = 27,59%
+ Fe2O3
- %mFe = \(\frac{56.2}{56.2+16.3}.100\%=70\%\)
- %mO = 100% - 70% = 30%
c/
+SO2
- %mS = \(\frac{32}{32+16.2}.100\%=50\%\)
- %mO = 100% - 50% = 50%
+ SO3
- %mS = \(\frac{32}{32+16.3}.100\%=40\%\)
- %mO = 100% - 40% = 60%
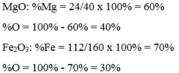
Ta có : % Fe = \(\frac{56.2}{56.2+16.3}.100\%=70\%\)
% O = 100% - 70%=30%
%mFe = \(\frac{56.2}{56.2+16.3}\) x 100% = 70%
%mO = 100% - 70% = 30%