Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Bài 1:
PTHH: \(C_2H_5OH+O_2\xrightarrow[]{mengiấm}CH_3COOH+H_2O\)
Ta có: \(n_{C_2H_5OH}=\dfrac{115\cdot0,8}{46}=2\left(mol\right)=n_{CH_3COOH\left(lýthuyết\right)}\)
\(\Rightarrow m_{CH_3COOH\left(thực\right)}=2\cdot60\cdot90\%=108\left(g\right)\)
Bài 2:
PTHH: \(C_2H_5OH+CH_3COOH\xrightarrow[H_2SO_4\left(đ\right)]{t^o}CH_3COOC_2H_5+H_2O\)
Ta có: \(\left\{{}\begin{matrix}n_{CH_3COOH}=\dfrac{60}{60}=1\left(mol\right)\\n_{C_2H_5OH}=\dfrac{92}{46}=2\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\) Rượu còn dư, Axit p/ứ hết
\(\Rightarrow n_{CH_3COOC_2H_5\left(lýthuyết\right)}=1\left(mol\right)\) \(\Rightarrow m_{CH_3COOC_2H_5\left(thực\right)}=1\cdot88\cdot80\%=70,4\left(g\right)\)

\(C_2H_5OH+O_2\rightarrow\left(men.giấm\right)CH_3COOH+H_2O\\ V_{C_2H_5OH}=25.4\%=1\left(l\right)=1000\left(ml\right)\\ m_{C_2H_5OH}=1000.0,8=800\left(g\right)\\ m_{CH_3COOH\left(LT\right)}=\dfrac{800.60}{46}=\dfrac{48000}{46}\left(g\right)\\ m_{CH_3COOH\left(TT\right)}=\dfrac{48000}{46}:92\%=1134,2155\left(gam\right)\\ m_{ddCH_3COOH}=1135,2155:5\%=22684,31\left(g\right)\)

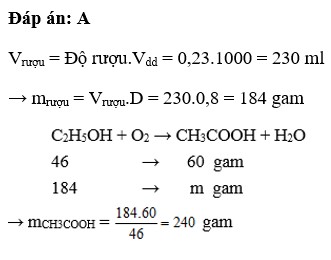
V C 2 H 5 OH = 50.4/100 = 2l
→ m C 2 H 5 OH = 2.1000.0,8 = 1600g
Phương trình hóa học :
C 2 H 5 OH + O 2 → CH 3 COOH + H 2 O
46 gam 60 gam
1600 gam x
x = 1600x60/46
Vì hiệu suất đạt 80% → m CH 3 COOH = 1600.60.80/(46.100) = 1669,6g
→ m giấm = 1669,6/5 x 100 = 33392 (gam) = 33,392 kg

a)
$C_2H_5OH + O_2 \xrightarrow{men\ giấm} CH_3COOH + H_2O$
V rượu = 57,5.12/100 = 6,9(lít) = 6900(cm3)
=> m rượu = 6900.0,8 = 5520(gam)
Theo PTHH :
n CH3COOH = n C2H5OH = 5520/46 = 120(mol)
m CH3COOH = 120.60 = 7200(gam)
b)
m dd giấm = 7200/4% = 180 000(gam)
\(V_r=57.5\cdot0.12=6.9\left(l\right)\)
\(m_{C_2H_5OH}=6.9\cdot0.8=5.52\left(g\right)\)
\(n_{C_2H_5OH}=\dfrac{5.52}{46}=0.12\left(mol\right)\)
\(n_{C_2H_5OH\left(pư\right)}=0.12\cdot92\%=0.1104\left(mol\right)\)
\(C_2H_5OH+O_2\underrightarrow{mg}CH_3COOH+H_2O\)
\(0.1104........................0.1104\)
\(m_{dd_{CH_3COOH}}=\dfrac{0.1104\cdot60}{4\%}=165.6\left(g\right)\)

Sơ đồ phản ứng
C 6 H 12 O 6 → 2 C 2 H 5 OH → 2 CH 3 COOH
Theo sơ đồ ta có: Cứ 180g glucozo thì tạo ra: 2.60 = 120g CH 3 COOH
Theo đề bài 50g glucozo ⇒ m CH 3 COOH thu được là: 50.120/180 = 33,33g
H = 60% ⇒ m CH 3 COOH = 33,33.60% = 20g
Khối lượng giấm ăn 4% = 20 : 4% = 50

a, \(V_{C_2H_5OH}=\dfrac{10.9}{100}=0,9\left(l\right)=900\left(ml\right)\)
\(\Rightarrow m_{C_2H_5OH}=900.0,8=720\left(g\right)\Rightarrow n_{C_2H_5OH}=\dfrac{720}{46}=\dfrac{360}{23}\left(mol\right)\)
PT: \(C_2H_5OH+O_2\underrightarrow{^{mengiam}}CH_3COOH+H_2O\)
Theo PT: \(n_{CH_3COOH\left(LT\right)}=n_{C_2H_5OH}=\dfrac{360}{23}\left(mol\right)\)
Mà: H = 92%
\(\Rightarrow n_{CH_3COOH\left(TT\right)}=\dfrac{360}{23}.92\%=14,4\left(mol\right)\)
\(\Rightarrow m_{CH_3COOH}=14,4.60=864\left(g\right)\)
b, \(m_{ddgiam}=\dfrac{864}{5\%}=17280\left(l\right)\)

a.\(V_{C_2H_5OH}=\dfrac{10.8}{100}=0,8ml\)
\(m_{C_2H_5OH}=0,8.0,8=1,6g\)
\(n_{C_2H_5OH}=\dfrac{1,6}{46}=0,034mol\)
\(C_2H_5OH+O_2\rightarrow\left(men.giấm\right)CH_3COOH+H_2O\)
0,034 0,034 ( mol )
\(m_{CH_3COOH}=0,034.60.80\%=1,632g\)
b.\(m_{dd_{CH_3COOH}}=\dfrac{1,632}{5\%}=32,64g\)

C2H5OH + O2 -> (lên men giấm) CH3COOH + H2O
nCH3COOH (LT) = nC2H5OH = 0,1 (mol)
nCH3COOH (TT) = 0,1 . 75% = 0,075 (mol)
mCH3COOH = 0,075 . 60 = 4,5 (g)
Thằng chó ngu người.