Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\((C_6H_{10}O_5)_n + nH_2O \xrightarrow{H^+} nC_6H_{12}O_6\\ C_6H_{12}O_6 \xrightarrow{men\ rượu,30^0-35^o}2CO_2 + 2C_2H_5OH\)
Theo PTHH :
\(n_{C_6H_{12}O_6} = n.n_{tinh\ bột\ pư} = n.\dfrac{100}{162n}.80\%= \dfrac{40}{81}mol\)
\(n_{C_6H_{12}O_6\ pư} = \dfrac{40}{81}.75\% = \dfrac{10}{27}mol\\ \Rightarrow n_{C_2H_5OH} = 2n_{C_6H_{12}O_6\ pư} = 2. \dfrac{10}{27}= \dfrac{20}{27}mol\\ \Rightarrow m_{C_2H_5OH} = \dfrac{20}{27}.46 = 34,074(kg)\)

a)n glucozo = 90/180 = 0,5(kmol)
n glucozo pư = 0,5.70% = 0,35(kmol)
$C_6H_{12}O_6 \xrightarrow{t^o,xt} 2CO_2 + 2C_2H_5OH$
n C2H5OH = 2n glucozo = 0,35.2 = 0,7(kmol)
m C2H5OH = 0,7.46 = 32,2(kg)
b)$(C_6H_{10}O_5)_n + nH_2O \xrightarrow{t^o,xt}nC_6H_{12}O_6$
$C_6H_{12}O_6 \xrightarrow{t^o,xt} 2CO_2 + 2C_2H_5OH$
n tinh bột = 2/162n = 1/81n(kmol)
n glucozo = 80% . n . 1/81n = 4/405(kmol)
n C2H5OH = 80% . 2. 4/405 = 32/2025(kmol)
m C2H5OH = 46.32/2025 = 0,73(kg)
\(n_{C_6H_{12}O_6}=\dfrac{90}{180}=0.5\left(kmol\right)\)
\(n_{C_6H_{12}O_6\left(pư\right)}=0.5\cdot0.7=0.35\left(kmol\right)\)
\(C_6H_{12}O_6\underrightarrow{^{\text{men rượu}}}2C_2H_5OH+2CO_2\)
\(0.35........................0.7\)
\(m_{C_2H_5OH}=0.7\cdot46=32.2\left(kg\right)\)
\(b.\)
\(C_{12}H_{22}O_{11}\underrightarrow{^{t^0,xt}}C_6H_{12}O_6+C_6H_{12}O_6\)
\(C_6H_{12}O_6\underrightarrow{^{\text{men rượu}}}2C_2H_5OH+2CO_2\)
\(n_{C_2H_5OH}=\dfrac{12\cdot n_{C_{12}H_{22}O_{11}}}{2}\cdot80\%=\dfrac{12\cdot\dfrac{1}{171}}{2}\cdot80\%=\dfrac{8}{285}\left(kmol\right)\)
\(m_{C_2H_5OH}=\dfrac{8}{285}\cdot46=1.29\left(kg\right)\)

(-C6H10O5-)n\(+nH_2O\overset{t^0}{\rightarrow}nC_6H_{12}O_6\)
C6H12O6\(\overset{t^0}{\rightarrow}2C_2H_5OH+2CO_2\)
\(\rightarrow\)(-C6H10O5-)n\(\overset{t^0}{\rightarrow}2nC_2H_5OH+2nCO_2\)
-Cứ 162n gam tinh bột tạo ra 92n gam rượu etylic
Vậy 106 gam tinh bột tạo ra x gam rượu etylic
x=\(\dfrac{10^6.92}{162}gam\)
Vì gạo có 80% tinh bột và hiệu suất quá trình là 60% nên:
\(V_{C_2H_5OH}=\dfrac{10^6\dfrac{92}{162}}{0,8}.\dfrac{80}{100}.\dfrac{60}{100}\approx0,34.10^6ml=0,34m^3\)

a)
\((C_6H_{10}O_5)_n + nH_2O \xrightarrow{t^o,xt} nC_6H_{12}O_6\\ C_6H_{12}O_6 \xrightarrow{t^o,xt} 2CO_2 + 2C_2H_5OH\\ \)
m tinh bột = 1.75% = 0,75(kmol)
n tinh bột = 0,75/162n = 1/216n(kmol)
n glucozo = n. 1/216n .81% = 3/800(kmol)
n C2H5OH = 2n glucozo . 81% = 243/40000(kmol)
m C2H5OH = 46.243/40000 = 0,27945(kg) = 279,45(gam)
V C2H5OH = 279,45/0,8 = 349,3125(ml)
V rượu 30o = 349,3125.100/30 = 1164,375(ml)
b)
$C_2H_5OH + O_2 \xrightarrow{men\ giấm} CH_3COOH + H_2O$
n CH3COOH = 243/40000 .92% = 5,6.10^-3(kmol)
m CH3COOH = 60.5,6.10^-3 = 0,336(kg) = 336(gam)

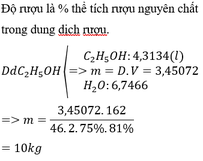
Đổi 10kg = 10000g
Ta có: \(n_{CH_3COOH\left(LT\right)}=\dfrac{10000.5\%}{92\%}=\dfrac{12500}{23}\left(mol\right)\)
PTHH:
\(C_2H_5OH+O_2\xrightarrow[]{\text{men giấm}}CH_3COOH+H_2O\)
\(\dfrac{12500}{23}\)<---------------------\(\dfrac{12500}{23}\)
\(\Rightarrow m_{C_2H_5OH}=\dfrac{12500}{23}.46=25000\left(g\right)=25\left(kg\right)\)

Ta có glucozo → 2C2H5OH + 2CO2
nrượu = 100 . 0,9 . 0,8 : 46 = 1,565 mol
=> mglucozo = 1,565 : 2 : 0,90 . 180 = 156,5 kg
\(V_{C_2H_5OH}=100.1000.90\%=9000\left(ml\right)\\ m_{C_2H_5OH}=9000.0,8=7200\left(g\right)\\ n_{C_2H_5OH}=\dfrac{7200}{46}=\dfrac{3600}{23}\left(mol\right)\)
PTHH: C6H12O6 -men rượu-> CO2 + C2H5OH
\(m_{C_6H_{12}O_6}=\dfrac{180.\dfrac{3600}{23}}{90\%}=\dfrac{720000}{23}\left(g\right)\)
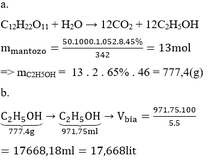
mC6H12O6= 8kg
C6H12O6-mr-> 2C2H5OH + 2CO2
180_____________92
8________________x
x= 8*92/180=4.08kg
Khối lượng C2H5OH thu được thực: 4.08*75/100=3.06kg