Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

1.\(n_{Fe_3O_4}=\dfrac{2,32}{232}=0,01mol\)
\(3Fe+2O_2\rightarrow\left(t^o\right)Fe_3O_4\)
0,3 0,1 ( mol )
\(m_{Fe}=0,3.56=16,8g\)
2.\(n_{Cu}=\dfrac{3,2}{64}=0,05mol\)
\(2Cu+O_2\rightarrow\left(t^o\right)2CuO\)
0,05 0,05 ( mol )
\(m_{CuO}=0,05.80=4g\)
3.\(n_{Na}=\dfrac{4,6}{23}=0,2mol\)
\(4Na+O_2\rightarrow\left(t^o\right)2Na_2O\)
0,2 0,05 ( mol )
\(V_{O_2}=0,05.24,79=1,2395l\)
4.\(n_{Cu}=\dfrac{1,6}{64}=0,025mol\)
\(2Cu+O_2\rightarrow\left(t^o\right)2CuO\)
0,025 0,0125 ( mol )
\(V_{O_2}=0,0125.24,79=0,309875l\)

\(n_{Fe}=\dfrac{8,4}{56}=0,15\left(mol\right)\\ pthh:3Fe+2O_2\underrightarrow{t^o}Fe_3O_{\text{4}}\)
0,15 0,1 0,05
\(m_{Fe_2O_4}=0,05.232=11,6\left(g\right)\\
V_{O_2}=0,1.11,4=2,24\left(l\right)\\
pthh:2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\uparrow\)
0,2 0,1
\(m_{KMnO_4}=0,2.158=31,6\left(g\right)\)
\(n_{Fe}=\dfrac{8,4}{56}=0,15\left(mol\right)\\ pthh:3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\)
0,15 0,1 0,05
\(m_{Fe_3O_{\text{ 4}}}=0,05.232=11,6\left(g\right)\\ V_{O_2}=0,1.22,4=2,24\left(l\right)\\ pthh:2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\)
0,1 0,05
\(m_{KMnO_4}=0,1.158=15,8\left(g\right)\)

\(a,n_{Fe}=\dfrac{16,8}{56}=0,3\left(mol\right)\\ 3Fe+2O_2\rightarrow\left(t^o\right)Fe_3O_4\\ b,n_{Fe_3O_4}=\dfrac{n_{Fe}}{3}=\dfrac{0,3}{3}=0,1\left(mol\right)\\ \Rightarrow m_{Fe_3O_4}=232.0,1=23,2\left(g\right)\\ n_{O_2}=\dfrac{2}{3}.n_{Fe}=\dfrac{2}{3}.0,3=0,2\left(mol\right)\\ \Rightarrow V_{O_2\left(đktc\right)}=0,2.22,4=4,48\left(l\right)\)

a. \(n_{Fe}=\dfrac{16,8}{56}=0,3\left(mol\right)\)
PTHH : 3Fe + 2O2 -to> Fe3O4
0,3 0,2 0,1
b. \(m_{Fe_3O_4}=0,1.232=23,2\left(g\right)\)
c. \(V_{O_2}=0,2.22,4=4,48\left(l\right)\)
a \(3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\)
b \(\Rightarrow n_{Fe}=\dfrac{16,8}{56}=0,3mol\) \(\Rightarrow n_{Fe_3O_4}=\dfrac{1}{3}n_{Fe}=0,1mol\Rightarrow m_{Fe_3O_4}=0,1\cdot232=2,32g\)
c \(\Rightarrow n_{O_2}=\dfrac{2}{3}n_{Fe}=0,2mol\Rightarrow V_{O_2}=0,2\cdot22,4=4,48l\)

a) \(n_{Fe}=\dfrac{3,36}{56}=0,06\left(mol\right)\)
PTHH: 3Fe + 2O2 --to--> Fe3O4
0,06->0,04------->0,02
=> mFe3O4 = 0,02.232 = 4,64 (g)
b) VO2 = 0,04.22,4 = 0,896 (l)

\(n_{Fe}=\dfrac{11,2}{56}=0,2\left(mol\right)\)
\(3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\)
0,2 2/15 1/15 ( mol )
\(V_{kk}=V_{O_2}.5=\left(\dfrac{2}{15}.22,4\right).5=14,93l\)
\(m_{Fe_3O_4}=\dfrac{1}{15}.232=15,46g\)
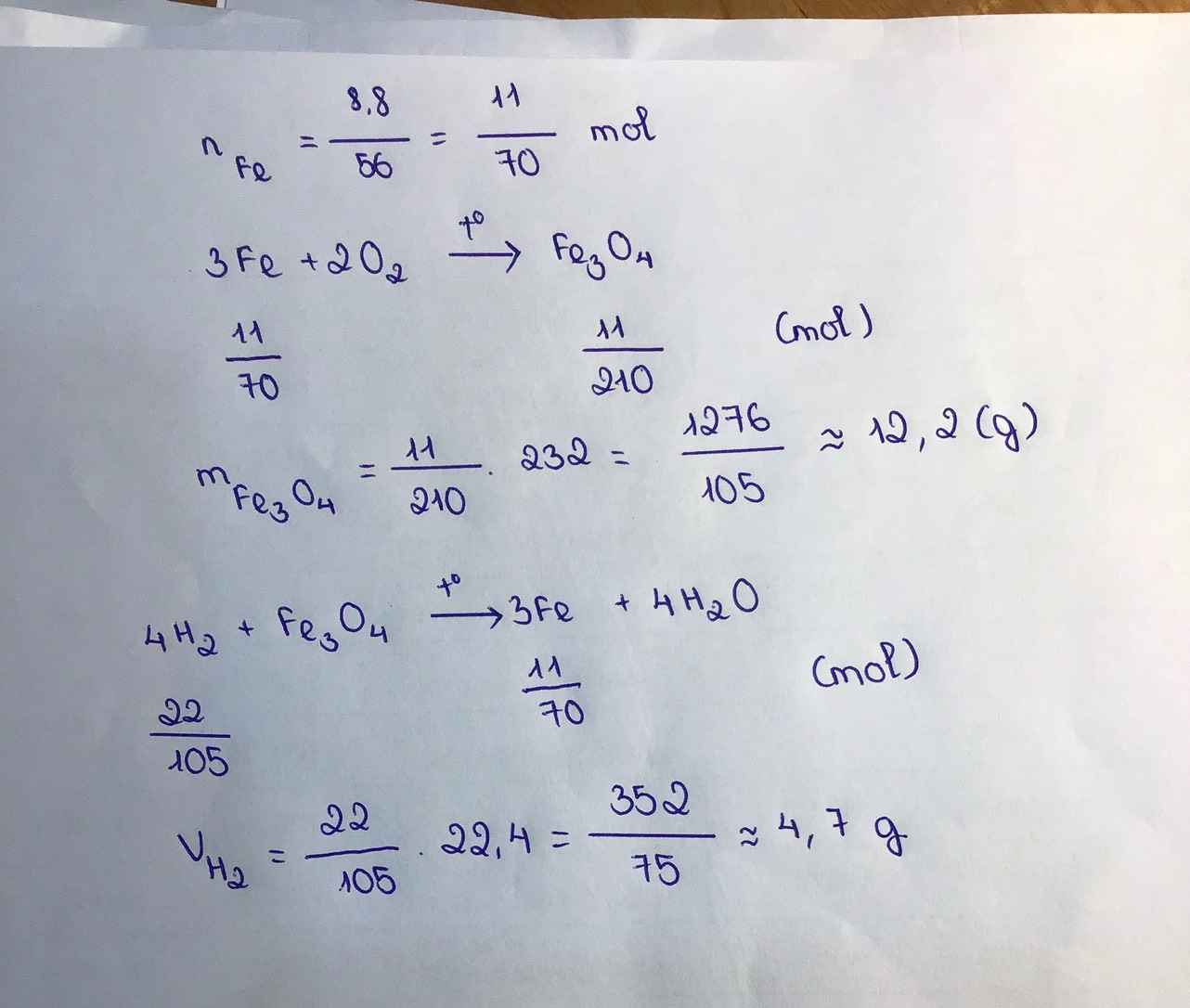

\(3Fe+2O_2\overset{t^0}{\rightarrow}Fe_3O_4\)
0,3 0,2 0,1
số mol Fe là: \(n=\dfrac{m}{M}=\dfrac{16,8}{56}=0,3\left(mol\right)\)
a. khối lượng oxit sắt sinh ra là:
\(m_{Fe_3O_4}=nM=0,1\cdot232=23,2\left(g\right)\)
b. thể tích khí O2 cần dùng là:
\(V_{O_2}=24,79\cdot n=24,79\cdot0,2=4,958\left(L\right)\)