Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(\left(4\right)CuO+H_2SO_4\rightarrow CuSO_4+H_2O\)
\(K_2O,BaO,Na_2O,CaO,Li_2O\) mới td đc với oxit axit chứ a
\(\left(1\right)CuO+2HCl\rightarrow CuCl_2+H_2O\\ \left(2\right)CuCl_2+2KOH\rightarrow Cu\left(OH\right)_2\downarrow+2KCl\\ \left(3\right)Cu\left(OH\right)_2\rightarrow\left(t^o\right)CuO+H_2O\\ \left(4\right)CuO+H_2SO_4\rightarrow CuSO_4+H_2O\)

a)
(1) \(Cu+2H2SO4\left(đ\right)-^{t0}->CuSO4+SO2\uparrow+2H2O\)
\(\left(2\right)CuSO4+BaCl2->CuCl2+BaSO4\downarrow\)
(3) \(CuCl2+2AgNO3->Cu\left(NO3\right)2+2AgCl\downarrow\)
(4) \(Cu\left(NO3\right)2+2NaOH->Cu\left(OH\right)2\downarrow+2NaNO3\)
(5) \(Cu\left(OH\right)2-^{t0}->CuO+H2O\)
(6) \(CuO+2HCl->CuCl2+H2O\)
(7) \(CuCl2-^{t0}->Cu+Cl2\uparrow\)
(8) \(Cu+Cl2-^{t0}->CuCl2\)
(9) \(CuCl2+2NaOh->Cu\left(OH\right)2\downarrow+2NaCl\)
b)
\(4Na+O2-^{t0}->2Na2O\)
\(Na2O+H2O->2NaOH\)
\(2NaOH+CO2->Na2CO3+H2O\)
\(Na2CO3+H2SO4->Na2SO4+CO2\uparrow+H2O\)
\(Na2SO4+BaCl2->2NaCl+BaSO4\downarrow\)
\(2NaCl+2H2O-\xrightarrow[có-màng-ngăn]{đpdd}2NaOH+Cl2\uparrow+H2\uparrow\)
\(3NaOH+H3PO4->Na3PO4+3H2O\)

\(CuO+H_2SO_4\rightarrow CuSO_4+H_2O\\ CuSO_4+Ba\left(NO_3\right)_2\rightarrow BaSO_4+Cu\left(NO_3\right)_2\\ Cu\left(NO_3\right)_2+2KOH\rightarrow Cu\left(OH\right)_2+2KNO_3\\ Cu\left(OH\right)_2\rightarrow\left(t^o\right)CuO+H_2O\\ CuO+2HCl\rightarrow CuCl_2+H_2O\\ CuCl_2\rightarrow\left(t^o\right)Cu+Cl_2\)

a) Fe2O3 + 6HCl -> 2FeCl3 + 3H2O
FeCl3 + 3NaOH -> Fe(OH)3 + 3NaCl
2Fe(OH)3 + 3H2SO4 -> Fe(SO4)3 + 6H2O
b) CuO + HCl -> CuCl2 + H2O
CuCl2 + 2NaOH->Cu(OH)2 + 2NaCl
Cu(OH)2 + H2SO4 -> CuSO4 +2H2O
a) \(Fe_2O_3\underrightarrow{\left(1\right)}FeCl_3\underrightarrow{\left(2\right)}Fe\left(OH\right)_3\underrightarrow{\left(3\right)}Fe\left(SO_{\text{4}}\right)_3\)
\(\left(1\right)2Fe_2O_3+6Cl_2\rightarrow4FeCl_3+3O_2\)
\(\left(2\right)FeCl_3+3NaOH\rightarrow Fe\left(OH\right)_3+3NaCl\)
\(\left(3\right)PTHH:??\)
b) \(CuO\underrightarrow{\left(1\right)}CuCl_2\underrightarrow{\left(2\right)}Cu\left(OH\right)_2\underrightarrow{\left(3\right)}CuSO_4\)
\(\left(1\right)CuO+2HCl\rightarrow CuCl_2+H_2O\)
\(\left(2\right)CuCl_2+2NaOH\rightarrow Cu\left(OH\right)_2+2NaCl\)
\(\left(3\right)Cu\left(OH\right)_2+H_2SO_4\rightarrow CuSO_4+2H_2O\)

\(Cu+O_2\underrightarrow{t^0}CuO\)
\(CuO+H_2SO_4\rightarrow CuSO_4+H_2O\)
\(CuSO_4+BaCl_2\rightarrow CuCl_2+BaSO_4\downarrow\)
\(CuCl_2+2KOH\rightarrow Cu\left(OH\right)_2\downarrow+2KCl\)

\(\text{CuO+2HCl(dung dịch pha loãng)=CuCl2+H2O}\)
\(\text{Ca(OH)2+CuCl2→Cu(OH)2↓+CaCl2}\)
\(\text{Cu(OH)2 → CuO + H2O}\)
\(\text{CuO + H2SO4 → H2O + CuSO4}\)
\(\text{CuSO4(dung dịch pha loãng)+H2=Cu↓+H2SO4}\)
PT cuối sai
\(CuO+2HCl->CuCl_2+H_2O\)
\(CuCl_2+2NaOH->Cu\left(OH\right)_2+2NaCl\)
\(Cu\left(OH\right)_2->CuO+H_2O\) (Nhiệt độ nữa nha bạn)
\(CuO+H_2SO_4->CuSO_4+H_2O\)
\(CuSO_4\:+Fe->FeSO_4+Cu\)
\(Cu+2AgNO_3->Cu\left(NO_3\right)_2+2Ag\)

$Cu(OH)_2 \xrightarrow{t^o} CuO + H_2O$
$CuO + 2HCl \to CuCl_2 + H_2o$
$CuCl_2 + 2AgNO_3 \to Cu(NO_3)_2 + 2AgCl$
$Cu(NO_3)_2 + 2KOH \to Cu(OH)_2 + 2KNO_3$
giúp e câu này với ah
Câu 11: Nhiệt phân hoàn toàn 30,3 gam hỗn hợp 2 bazơ không tan Cu(OH)2 và Fe(OH)3, sau pư thu được 24 gam 2 oxit.
a) Tính % số mol mỗi bazơ trong hỗn hợp đầu?
Cho 2 oxit trên pư vừa đủ với 200 ml dung dịch HCl xM. Tìm x?
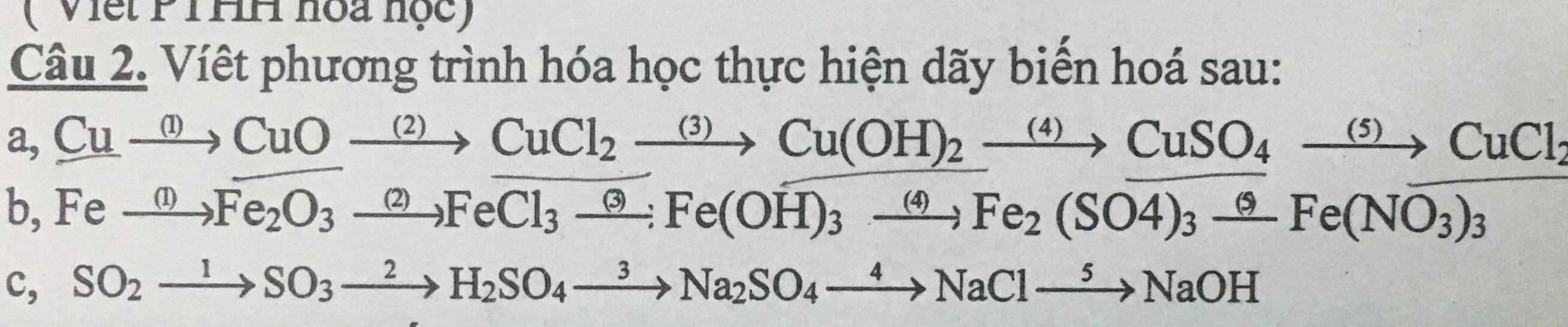
\(CuO+2HCl\rightarrow CuCl_2+H_2O\)
\(CuCl_2+Ca\left(OH\right)_2\rightarrow Cu\left(OH\right)_2\downarrow+CaCl_2\)
\(Cu\left(OH\right)_2\rightarrow CuO+H_2O\)
\(CuO+H_2SO_4\rightarrow CuSO_4+H_2O\)
\((1)CuO+2HCl\to CuCl_2+H_2O\\ (2)CuCl_2+2NaOH\to Cu(OH)_2\downarrow+2NaCl\\ (3)Cu(OH)_2\xrightarrow{t^o}CuO+H_2O\\ (4)CuO+H_2SO_4\to CuSO_4+H_2O\)