Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

PTHH: CuO + CO --to--> Cu + CO2
______0,1---------------------->0,1
Fe2O3 + 3CO --> 2Fe + 3CO2
_0,1----------------------->0,3
=> VCO2 = (0,1+0,3).22,4 = 8,96(l)
=> D

a)
Gọi $n_{CO_2} = a(mol) \Rightarrow n_{CO} = 2a(mol)$
$n_C = 0,3(mol)$
Bảo toàn nguyên tố C :
$n_{CO_2} + n_{CO} =n_C \Rightarrow a + 2a = 0,3 \Rightarrow a = 0,1$
$V_{CO_2} = 0,1.22,4 = 2,24(lít)$
$V_{CO} = 0,2.22,4 = 4,48(lít)$
b)
Bảo toàn O :
$2n_O = 2n_{CO_2} + n_{CO} \Rightarrow n_{O_2} = \dfrac{0,1.2 + 0,2}{2} = 0,2(mol)$
$V_{O_2} = 0,2.22,4 = 4,48(lít)$

a) PTHH: \(CuO+CO\xrightarrow[]{t^o}Cu+CO_2\uparrow\)
a_____a (mol)
\(Fe_2O_3+3CO\xrightarrow[]{t^o}2Fe+3CO_2\uparrow\)
b______3b (mol)
Ta lập HPT: \(\left\{{}\begin{matrix}80a+160b=40\\a+3b=\dfrac{15,68}{22,4}=0,7\end{matrix}\right.\) \(\Leftrightarrow\left\{{}\begin{matrix}a=0,1\\b=0,2\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{CuO}=\dfrac{0,1\cdot80}{40}\cdot100\%=20\%\\\%m_{Fe_2O_3}=80\%\end{matrix}\right.\)
b) PTHH: \(CuO+H_2SO_4\rightarrow CuSO_4+H_2O\)
0,1______0,1 (mol)
\(2Fe_2O_3+3H_2SO_4\rightarrow Fe_2\left(SO_4\right)_3+3H_2O\)
0,2_______0,3 (mol)
Ta có: \(n_{H_2SO_4}=0,4\left(mol\right)\) \(\Rightarrow m_{ddH_2SO_4}=\dfrac{0,4\cdot98}{10\%}=392\left(g\right)\)
a)Gọi x,y lần lượt là số mol CuO, Fe2O3
| CO | + | CuO | ⟶ | Cu | + | CO2 |
Fe2O3 + 3CO → 2Fe + 3CO2
\(\left\{{}\begin{matrix}80x+160y=40\\x+3y=0,7\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}x=0,1\\y=0,2\end{matrix}\right.\)
=> \(\%m_{CuO}=\dfrac{80.0,1}{40}.100=20\%\)
=> %mFe2O3 = 80%
b) \(CuO+H_2SO_4\rightarrow CuSO_4+H_2O\)
\(Fe_2O_3+3H_2SO_4\rightarrow Fe_2\left(SO_4\right)_3+3H_2O\)
\(n_{H_2SO_4}=0,7\left(mol\right)\)
=> \(m_{ddH_2SO_4}=\dfrac{0,7.98}{10\%}=686\left(g\right)\)

\(n_{NaOH}=\dfrac{16.10\%}{40}=0,04\left(mol\right)\\ 2NaOH+SO_2\rightarrow Na_2SO_3+H_2O\\ NaOH+SO_2\rightarrow NaHSO_3\\ Đặt:n_{NaHSO_3}=a\left(mol\right);n_{Na_2SO_3}=1,5a\left(mol\right)\\ \Rightarrow n_{NaOH\left(tổng\right)}=3a+a=4a=0,04\left(mol\right)\\ \Leftrightarrow a=0,01\left(mol\right)\\ n_{SO_2\left(tổng\right)}=n_{Na_2SO_3}+n_{NaHSO_3}=2,5a=0,025\left(mol\right)\\ V_{SO_2\left(đktc\right)}=0,025.22,4=0,56\left(lít\right)\)

200ml = 0,2l
\(n_{Zn}=\dfrac{19,5}{65}=0,3\left(mol\right)\)
Pt : \(Zn+2HCl\rightarrow ZnCl_2+H_2|\)
1 2 1 1
0,3 0,6 0,3 0,3
a) \(n_{ZnCl2}=\dfrac{0,3.1}{1}=0,3\left(mol\right)\)
\(C_{M_{ZnCl2}}=\dfrac{0,3}{0,2}=1,5\left(M\right)\)
b) \(n_{H2}=\dfrac{0,3.1}{1}=0,3\left(mol\right)\)
\(V_{H2\left(dktc\right)}=0,3.22,4=6,72\left(l\right)\)
c) Pt : \(NaOH+HCl\rightarrow NaCl+H_2O|\)
1 1 1 1
0,6 0,6
\(n_{NaOH}=\dfrac{0,6.1}{1}=0,6\left(mol\right)\)
\(m_{NaOH}=0,6.40=24\left(g\right)\)
\(m_{ddNaOH}=\dfrac{24.100}{20}=120\left(g\right)\)
Chúc bạn học tốt

a.\(m_{dd.Br_2\left(tăng\right)}=m_{C_2H_2}=2,6g\)
\(n_{hh}=\dfrac{5,6}{22,4}=0,25mol\)
\(n_{C_2H_2}=\dfrac{2,6}{26}=0,1mol\)
\(\%V_{C_2H_2}=\dfrac{0,1}{0,25}.100=40\%\)
\(\%V_{CH_4}=100\%-40\%=60\%\)
b.\(C_2H_2+2Br_2\rightarrow C_2H_2Br_4\)
0,1 0,2 ( mol )
\(C_{M\left(dd.Br_2\right)}=\dfrac{0,2}{0,1}=2M\)

Khí thoát ra là metan
\(\%V_{CH_4} = \dfrac{5,6}{13,44}.100\% =41,67\%\\ \%V_{C_2H_4} = 100\% - 41,67\% = 58,33\%\\ b) V_{C_2H_2} = 13,44 -5,6 = 7,84(lít)\\ n_{C_2H_2} = \dfrac{7,84}{22,4} = 0,35(mol)\\ C_2H_2 + 2Br_2 \to C_2H_2Br_4\\ n_{Br_2} = 2n_{C_2H_2} = 0,7(mol)\\ \Rightarrow C_{M_{Br_2}} = \dfrac{0,7}{0,25} = 2,8M\\ c)\)
\(CH_4 + 2O_2 \xrightarrow{t^o} CO_2 + 2H_2O\\ C_2H_4 + 3O_2 \xrightarrow{t^o} 2CO_2 + 2H_2O\\ V_{O_2} = 2V_{CH_4} + 3V_{C_2H_4} = 5,6.2 + 7,84.3 = 34,72(lít)\)
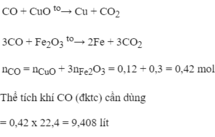
\(CuO+CO-->Cu+CO2\)
\(Fe2O3+3CO-->2Fe+3CO2\)
Theo pthh1
\(n_{CO}=n_{CuO}=0,12\left(mol\right)\)
Theo pthh2
\(n_{CO}=3n_{Fe2O3}=0,3\left(mol\right)\)
\(\sum n_{CO}=0,3+0,12=0,42\left(mol\right)\)
\(V_{CO}=0,42.22,4=9,408\left(l\right)\)
cảm ơn ạ