Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

2KClO3-to\xt->2KCl+3O2
0,1------------------0,1
n KClO3=\(\dfrac{12,25}{122,5}\)=0,1 mol
=>m KCl=0,1.74,5=7,45g
H=\(\dfrac{6,8}{7,45}.100\)=91,275%
b)
2KClO3-to\xt->2KCl+3O2
0,2-------------------------0,3 mol
n O2=\(\dfrac{6,72}{22,4}\)=0,3 mol
H=85%
=>m KClO3=0,2.122,5.\(\dfrac{100}{85}\)=28,82g
c)
2KClO3-to\xt->2KCl+3O2
0,2------------------------0,3
n KClO3=\(\dfrac{24,5}{122,5}\)=0,2 mol
H=80%
=>m O2=0,3.32.\(\dfrac{80}{100}\)=10,4g

a)
\(n_{Mg} = \dfrac{3,6}{24} = 0,15(mol)\\ 2Mg + O_2 \xrightarrow{t^o} 2MgO\\ n_{O_2} = \dfrac{1}{2}n_{Mg} = 0,075(mol)\\ \Rightarrow V_{O_2} = 0,075.22,4 = 1,68(lít)\)
b)
\(2KClO_3 \xrightarrow{t^o,MnO_2} 2KCl + 3O_2\\ n_{KClO_3} = \dfrac{2}{3}n_{O_2} = \dfrac{2}{3}.0,075 = 0,05(mol)\\ \Rightarrow m_{KClO_3} = 0,05.122,5 = 6,125(gam)\)

Câu 2:
a. PTHH:\(2KClO_3\rightarrow2KCl+3O_2\)
b. \(n_{KClO_3}=\frac{36,75}{122,5}=0,3\left(mol\right)\)
Theo PTHH: \(n_{O_2}=\frac{3}{2}n_{KClO_3}=\frac{3}{2}.0,3=0,45\left(mol\right)\)
\(\Rightarrow m_{O_2}=0,45.32=14,4\left(g\right)\)
\(\Rightarrow V_{O_2}=0,45.22,4=10,08\left(l\right)\)
c. *Cách 1: Tính theo KClO3
Theo PTHH: \(n_{KCl}=n_{KClO_3}=0,3\left(mol\right)\)
\(\Rightarrow m_{KCl}=0,3.74,5=22,35\left(g\right)\)
* Cách 2: Tính theo O2
Theo PTHH:\(n_{KCl}=\frac{2}{3}n_{O_2}=\frac{2}{3}.0,45=0,3\left(mol\right)\)
\(\Rightarrow m_{KCl}=0,3.74,5=22,35\left(g\right)\)
câu 2
a) 2KClO3--->2KCl+3O2
b) n KClO3=36,75/122,5=0,3(mol)
n O2=3/2n KClO3=0,45(mol)
m O2=0,45.32=14,4(g)
V O2=0,45.22,4=10,08(l)
c) Cách 1
Áp dụng ĐLBTKL
m KCl=m KClO3-m O2
=36,75-14,4=22,35(g)
Cách 2
n KCl=n KClO3=0,3(mol)
m KCl=0,3.74,5=22,35(g)
Bài 3
C+O2--->CO2
n C=6/12=0,5(mol)
n CO2=n C=0,5(mol)
V CO2=0,5.22,4=11,2(l)

a) \(2KClO_3 \xrightarrow{t^o} 2KCl + 3O_2\)
b)
\(n_{KClO_3} = \dfrac{36,75}{122,5} = 0,3(mol)\)
Theo PTHH :
\(n_{KCl} = n_{KClO_3} = 0,3(mol)\\ \Rightarrow m_{KCl} = 0,3.74,5 = 22,35(gam)\\ \Rightarrow m_{O_2} = m_{KClO_3} - m_{KCl} = 14,4(gam)\)
c)
Bảo toàn khối lượng :
\(m_{O_2} = 25 - 15,4 = 9,6(gam)\\ \Rightarrow n_{O_2} = \dfrac{9,6}{32} = 0,3(mol)\\ n_{KClO_3} = \dfrac{2}{3}n_{O_2} = 0,2(mol)\\ \Rightarrow m_{KClO_3} = 0,2.122,5 = 24,5(gam)\\ \%m_{tạp\ chất}= \dfrac{25-24,5}{25}.100\% = 2\%\)
\(a.\)
\(2KClO_3\underrightarrow{t^0}2KCl+3O_2\)
\(b.\)
\(n_{KClO_3}=\dfrac{36.75}{122.5}=0.3\left(mol\right)\)
\(\Rightarrow n_{O_2}=\dfrac{3}{2}n_{KClO_3}=\dfrac{3}{2}\cdot0.3=0.45\left(mol\right)\)
\(m_{O_2}=0.45\cdot32=14.4\left(g\right)\)
\(m_{KCl}=0.3\cdot74.5=22.35\left(g\right)\)
\(c.\)
\(2KClO_3\underrightarrow{t^0}2KCl+3O_2\)
\(a.............a\)
\(m_{Cr}=m_{KCl}+m_{tc}=25-122.5a+74.5a=15.4\left(g\right)\)
\(\Rightarrow a=0.2\)
\(m_{O_2}=\dfrac{3}{2}\cdot0.2\cdot32=9.6\left(g\right)\)
\(m_{KClO_3}=0.2\cdot122.5=24.5\left(g\right)\)
\(m_{tc}=25-24.5=0.5\left(g\right)\)
\(\%m_{Tc}=\dfrac{0.5}{25}\cdot100\%-2\%\)

1.
\(2KClO_3\underrightarrow{^{to}}2KCl+3O_2\)
\(n_{KClO3}=\frac{9,8}{122,5}=0,08\left(mol\right)\)
\(\Rightarrow n_{O2}=\frac{3}{2}n_{KClO3}=\frac{3}{2}.0,08=0,12\left(mol\right)\)
\(\Rightarrow V_{O2}=0,12.22,4=2,688\left(l\right)\)
2.
\(a,2KMnO_4\underrightarrow{^{to}}K_2MnO_4+MnO_2+O_2\)
\(b,n_{O2}=\frac{33,6}{22,4}=1,5\left(mol\right)\)
\(\Rightarrow n_{KMnO4}=2n_{O2}=2.1,5=3\left(mol\right)\)
\(\Rightarrow m_{KMnO4}=3.158=474\left(g\right)\)
3.
\(2KMnO_4\underrightarrow{^{to}}K_2MnO_4+MnO_2+O_2\)
1____________________________0,5
\(2KClO_3\underrightarrow{^{to}}2KCl+3O_2\left(1\right)\)
1____________________1,5
Đặt \(n_{KMnO4}=n_{KClO3}=1\left(mol\right)\)
\(V_{O2\left(1\right)}=0,5.22,4=11,2\left(l\right)\)
\(V_{O2\left(2\right)}=1,5.22,4=33,6\left(l\right)\)
Vậy nung KClO3 sẽ cho thể tích oxi nhiều hơn.

nO2 = 16,8 : 22,4 = 0,75 (mol)
2KMnO4 -t--> K2MnO4 + MnO2 + O2
1,5 <-------------------------------0,75(mol)
=> mKClO3 = 1,5 . 122,5 = 183,75 ( g)

Sửa đề: \(KClO_3\)
a) \(PTHH:2KClO_3\rightarrow2KCl+3O_2\)
b) Tỉ lệ: \(2:2:3\)
c) Theo định luật \(BTKL\) , ta có:
\(m_{KClO_3}=m_{KCl}+m_{O_2}\)
\(m_{KClO_3}=14,9+9,6\)
\(\Rightarrow m_{KClO_3}=24,5\left(g\right)\)
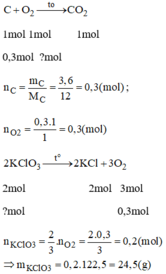
C+O2→CO2
1 mol →→ 1 mol
nC=3,6\12=0,3mol→0,3mol
Số mol khí oxi cần có là :
2KClO3to⟶2KCl+3O2
2 mol ---- →→--------- 3 mol
x mol ------←← -------0,3 mol
x=0,3×23=0,2(mol)
Khối lượng KClO3 cần dùng là: 0,2×122,5=24,5(g)